Description
Overview
The Acro Biotech COVID-19 Rapid POC CE-IVD test is a lateral flow immunoassay which qualitatively assess the presence of patient-generated IgG and IgM antibodies against SARS-CoV-2, the causative agent of the novel coronavirus disease COVID-19. The test cassette can detect these antibodies in whole blood, serum or plasma specimens.
| Pack Size | Tests |
1 pack | 25 tests |
Intended Use
A rapid test for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2 in human whole blood, serum or plasma specimens. For professional in vitro diagnostic use only. The COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2 in human whole blood, serum or plasma specimen.
Summary
Early January 2020, a novel coronavirus (SARS-CoV-2) was identified as the infectious agent causing an outbreak of viral pneumonia in Wuhan, China, where the first cases had their symptom onset in December 2019. Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases. Six coronavirus species are known to cause human disease. Four viruses — 229E, OC43, NL63, and HKU1 — are prevalent and typically cause common cold symptoms in immunocompetent individuals. The two other strains — severe acute respiratory syndrome coronavirus (SARS-COV) and Middle East respiratory syndrome coronavirus (MERS-COV) — are zoonotic in origin and have been linked to sometimes fatal illness. Coronaviruses are zoonotic, meaning they are transmitted between animals and people. Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death. Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
Biological Principle
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in IgG test line region. During testing, the specimen reacts with SARS-CoV-2 antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti-human IgG in IgG test line region, if the specimen contains IgG antibodies to 2019-nCoV. A colored line will appear in IgG test line region as a result of this.
Similarly, anti-human IgM is coated in IgM test line region and if specimen contains IgM antibodies to SARS-CoV-2, the conjugate-specimen complex reacts with anti-human IgM. A colored line appears in IgM test line region as a result. Therefore, if the specimen contains 2019-nCoV IgG antibodies, a colored line will appear in IgG test line region. If the specimen contains SARS-CoV-2 IgM antibodies, a colored line will appear in IgM test line region. If the specimen does not contain SARS-CoV-2 antibodies, no colored line will appear in either of the test line regions, indicating a negative result.
To serve as a procedural control, a colored line should always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
Reagents
The test contains anti-human IgM and anti-human IgG as the capture reagent, and SARS-CoV-2 antigen as the detection reagent. A goat anti-mouse IgG is employed in the control line system.
Precautions
- For professional in vitro diagnostic use only.
- Do not use after expiration date.
- Do not eat, drink or smoke in the area where the specimens or kits are handled.
- Do not use test if pouch is damaged.
- Handle all specimens as if they contain infectious agents.
- Observe established precautions against microbiological hazards throughout all procedures and follow the standard procedures for proper disposal of specimens.
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
- Please ensure that an appropriate amount of samples are used for testing. Too much or too little sample size may lead to deviation of results.
- The used test should be discarded according to local regulations.
- Humidity and temperature can adversely affect results.
Storage and Stability
Store as packaged in the sealed pouch at room temperature or refrigerated (2-30°C). The test is stable through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond the expiration date.
Specimen Collection and Preparation
The COVID-19 IgG/IgM Rapid test can be performed using whole blood (from venipuncture or fingerstick), serum or plasma.
To collect Fingerstick Whole Blood Specimens:
-
- Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow to dry.
- Massage the hand without touching the puncture site by rubbing down the hand towards the fingertip of the middle or ring finger.
- Puncture the skin with a sterile lancet. Wipe away the first sign of blood.
- Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site.
- Add the Fingerstick Whole Blood specimen to the test by using a capillary tube:
- Touch the end of the capillary tube to the blood until filled to approximately 20uL. Avoid air bubbles.
- Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
- Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up to 7 days, for long term storage, serum/plasma specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days of collection. Do not freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
- Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
- If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiological agents.
- EDTA K2, Heparin sodium, Citrate sodium and Potassium Oxalate can be used as the anticoagulant for collecting the specimen.
Materials
Provided:
- Test cassettes
- Droppers
- Package insert
- Buffer
Required, not provided:
- Specimen collection containers
- Lancets (for fingerstick whole blood only)
- Capillary tubes
- Centrifuge (for plasma only)
- Timer
- Pipette
Directions
Allow the test,, specimen, buffer and/or controls to reach room temperature (15-30°C) prior to testing.
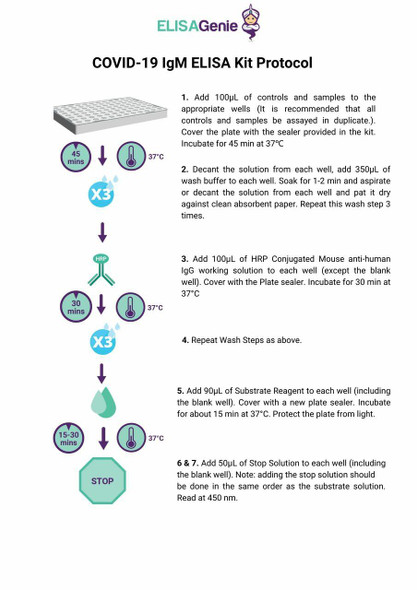
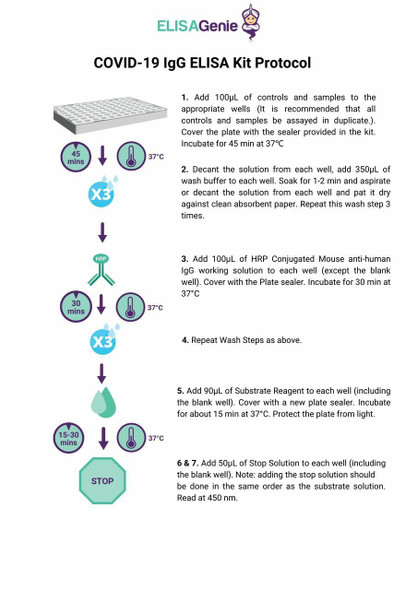
1. Remove the test cassette from the foil pouch and use it within one hour. Best results will be obtained if the test is performed immediately after opening the foil pouch.
2. Place the cassette on a clean and level surface.
For Serum or Plasma specimen:
To use a dropper: Hold the dropper vertically, draw the specimen to the fill line (approximately 10μL), and transfer the specimen to the specimen well (S), then add 2 drops of buffer (approximately 80 μL), and start the timer.
To use a pipette: To transfer 10 uL of specimen to the specimen well(S), then add 2 drops of buffer (approximately 80 μL), and start the timer.
For Venipuncture Whole Blood specimen:
To use a dropper: Hold the dropper vertically, draw the specimen about 1 cm above the fill line and transfer 1 full drop (approx. 20μL) of specimen to the sample well(S). Then add 2 drops of buffer (approximately 80 μL) and start the timer.
To use a pipette: To transfer 20 μL of whole blood to the specimen well(S), then add 2 drops of buffer (approximately 80 μL), and start the timer.
For Fingerstick Whole Blood specimen:
To use a dropper: Hold the dropper vertically, draw the specimen about 1 cm above the fill line and transfer 1 full drop (approx. 20μL) of specimen to the sample well(S). Then add 2 drops of buffer (approximately 80 μL) and start the timer.
To use a capillary tube: Fill the capillary tube and transfer approximately 20μL of fingerstick whole blood specimen to the specimen well (S) of test cassette, then add 2 drops of buffer (approximately 80 μL) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result until after 20 minutes.
Note: It is suggested not to use the buffer, beyond 6 months after opening the vial.
Fig 1. Test and results schematic.
Interpretation of Results
IgG POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the IgG line region.
IgM POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the IgM line region.
IgG and IgM POSITIVE:* Three colored lines appear. One colored line should always appear in the control line region (C) and two test lines should be in the IgG line region and IgM line region.
*NOTE: The intensity of the color in the test line regions may vary depending on the concentration of SARS-CoV-2 antibodies present in the specimen. Therefore, any shade of color in the test line region should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the IgG region and IgM region.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Quality Control
Internal procedural controls are included in the test. A colored line appearing in the control region (C) is an internal procedural control. It confirms sufficient specimen volume and correct procedural technique. Control standards are not supplied with this kit; however, it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
Limitations
1. The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is for in vitro diagnostic use only. This test should be used only for detection of IgG and IgM antibody to SARS-CoV-2 in whole blood, serum or plasma specimens. Neither the quantitative value nor the rate of increase in the concentration of IgG or IgM antibodies to SARS-CoV-2 can be determined by
this qualitative test.
2. The COVID-19 IgG/IgM Rapid Test Cassette (Whole blood/Serum/Plasma) will only indicate the presence of IgG and IgM antibodies to SARS-CoV-2 in the specimen and should not be used as the sole criteria for the diagnosis of SARS-CoV-2 infections.
3. As with all diagnostic tests, all results must be considered with other clinical information available to the physician.
4. If the test result is negative and clinical symptoms persist, additional follow-up testing using other clinical methods is suggested. A negative result at any time does not preclude the possibility of SARS-CoV-2 infection.
5. The hematocrit level of the whole blood can affect the test results. Hematocrit level needs to be between 25% and 65% for accurate results.
6. The test will show negative results under the following conditions: The titer of the novel coronavirus antibodies in the sample is lower than the minimum detection limit of the test, or the novel coronavirus antibody has not appeared at the time of sample collection (asymptomatic stage).
Performance Characteristics
Sensitivity and Specificity
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) was compared with a leading commercial PCR; the results show that the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) has high sensitivity and specificity.
Cross-Reactivity
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) has been tested for anti-influenza A virus, anti-influenza B virus, anti-RSV, anti-Adenovirus, HBsAg, anti-Syphilis, antiH. Pylori, anti-HIV and anti-HCV positive specimens. The results showed no cross-reactivity.
Interferring Substances
The following compounds have been tested using the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) and no interference was observed.
Triglyceride: 50 mg/dL
- Ascorbic Acid: 20mg/dL
- Hemoglobin 1000mg/dL
- Bilirubin: 60mg/dL
- Total cholesterol : 6mmol/L
Disclaimer
While we believe this kit is to be an effective indicator of infection we cannot guarantee 100% accuracy so the patient should still be advised to follow government guidelines for those exhibiting symptoms and those who aren’t regarding hygiene, self-isolation and other measures even if the test is negative.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- This kit is for Professional-Use-Only.