Therapeutic Drug Monitoring
Vedolizumab (Entyvio®) ELISA Kit
- SKU:
- HUMB00018
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Vedolizumab (Entyvio®)
- Research Area:
- Anti-Inflammatory
Description
Vedolizumab (Entyvio®) ELISA Kit
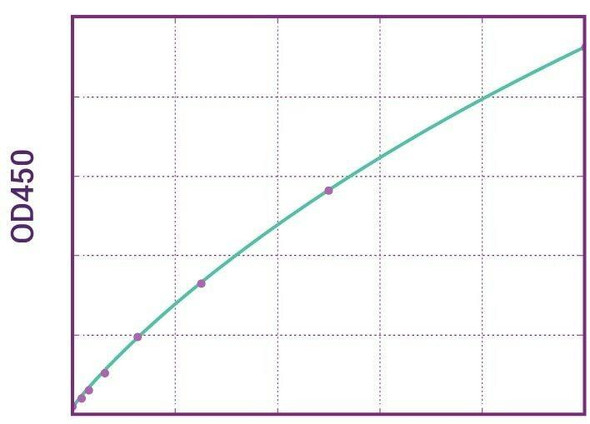
Enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of Vedolizumab (Entyvio®) in human serum and plasma. This Assay Genie kit has been especially developed for the quantitative analysis of Vedolizumab in serum and plasma samples and is for research use only.
Vedolizumab (Entyvio®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtitre plate coated with the reactant for vedolizumab (Entyvio®). After incubation, the wells are washed. A horse radish peroxidase (HRP) conjugated probe is added and binds to vedolizumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of vedolizumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Vedolizumab (Entyvio®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (Ž¼l) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
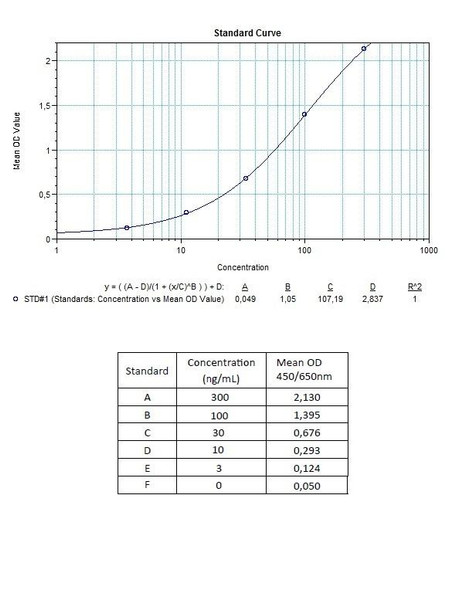
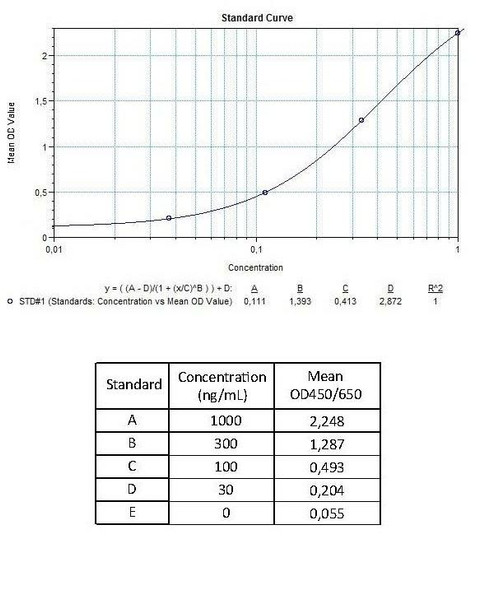
Detection Limit (ng/mL) | 30 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-Human lymphocyte α4β7 integrin Entyvio |
Vedolizumab (Entyvio®) - Key Information
Vedolizumab (Entyvio®) mode of action
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody directed against the human lymphocyte α4β7 integrin, a key mediator of gastrointestinal inflammation. Vedolizumab binds specifically to α4β7 integrin but does not bind to, or inhibit function of, α4β1 or αEβ7 integrins. Inhibition of the α4β7 integrin is a shared mechanism with natalizumab, however vedolizumab binds solely to the α4β7but not the α4β1 integrin, unlike natalizumab which binds to both.
As a result, natalizumab modulates the systemic immune system and is associated with other side effects such as progressive multifocal leukoencephalopathy (PML). Non-clinical studies have shown that the pharmacodynamic effects of vedolizumab are reversible upon removal of the antibody: pharmacologic activity of cells inhibited by vedolizumab could be partially restored within 24 hours after removal, with near complete restoration within 4 days. There are no known drug interactions as vedolizumab is a humanized antibody and does not modulate production of cytokines, which is known to affect drug metabolism.
Vedolizumab (Entyvio®) uses
It is used in the treatment of moderate to severe active ulcerative colitis and Crohn's disease for patients who have had an inadequate response with, lost response to, or were intolerant to inhibitors of tumor necrosis factor alpha (TNF alpha) or other conventional therapies. By blocking its primary target, α4β7 integrin, vedolizumab reduces inflammation in the gut. Vedolizumab binds to α4β7 integrin, a key mediator of gastrointestinal inflammation expressed on the surfaces of T and B lymphocytes. By selectively inhibiting the α4β7 integrin, vedolizumab inhibits adhesion of lymphocytes to its natural ligand, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), thereby preventing lymphocytic cells from entering the gut lamina propria and gut-associated lymphoid tissue (GALT). Specifically inhibiting this pathway alleviates GI inflammation without impairing systemic immune responses.
Vedolizumab (Entyvio®) treatment
Vedolizumab is indicated for adult patients with moderately to severely active ulcerative colitis or Crohn's disease who have had an inadequate response with, lost response to, or were intolerant to a tumor necrosis factor (TNF) blocker or immunomodulator; or had an inadequate response with, were intolerant to, or demonstrated dependence on corticosteroids.
Vedolizumab (Entyvio®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
7 x 0.3 mL | Vedolizumab Standards A-E (10x), High Level Control, Low Level Control Standard A: 1000 ng/mL Used for the standard curve and control. Contains vedolizumab, human serum and stabilizer, <0,1% NaN3. |
10 x 0.3 mL | Controls Low and high levels (10x) |
2 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Vedolizumab (Entyvio®) ELISA Protocol
| Steps | Protocol |
1 | Dilute each of the standards, controls and samples as described in “Pre-test |
2 | Pipette 100 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. |
3 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use Conugate into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Entyvio® is a trademark of Millennium Pharmaceuticals, Inc.