Description
The ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of samples in cell culture supernatant, serum, plasma (EDTA, citrate, heparin).
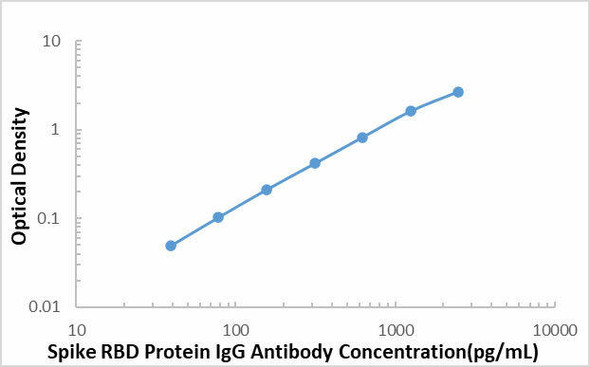
This SARS-COV-2 Spike RBD Protein Antibody ELISA kit is intended for laboratory research use only and not for use in diagnostic or therapeutic procedures. The stop solution changes color from blue to yellow and the intensity of the color is measured at 450 nm using a spectrophotometer. In order to measure the concentration of SARS-COV-2 Spike RBD Protein Antibody in the sample, this SARS-COV-2 Spike RBD Protein Antibody ELISA Kit includes a set of calibration standards. The calibration standards are assayed at the same time as the samples and allow the operator to produce a standard curve of optical density versus SARS-COV-2 Spike RBD Protein Antibody concentration. The concentration of the samples is then determined by comparing the O.D. of the samples to the standard curve.
| Information | Description |
| Product Name | SARS-COV-2 Spike RBD Protein Antibody ELISA Kit |
| SKU Number | CBK4144 |
| Size | 96T |
| Sample | 100 uL |
| Kit Components |
|
| Instruments | Microplate Reader |
| Detection | Colorimetric |
| Assay Time | 4 hours |
| Conjugate | Biotin |
| Precautions | Sandwich ELISA |
| Sensitivity | 121.4 pg/mL |
| Standard Curve Range | 391-25000 pg/mL |
| Storage Conditions | Store at 4°C |
Safety Notes
1. This kit contains materials with small quantities of sodium azide. Sodium azide reacts with lead and copper plumbing to form explosive metal azides. Upon disposal, flush drains with a large volume of water to prevent azide accumulation. Avoid ingestion and contact with eyes, skin or mucous membranes. In the case of contact, rinse the affected area with plenty of water. Observe all federal, state and local regulations for disposal.
2. All blood components and biological materials should be handled as potentially hazardous. Follow universal precautions as established by the Centers for Disease Control and Prevention and by the Occupational Safety and Health Administration when handling and disposing of infectious agents.