Description
| Product Name: | QuickStep Human MAU (Microalbuminuria) ELISA Kit |
| Product Code: | AEES00634 |
| Assay Type: | Sandwich |
| Format: | 96 Assays |
| Assay Time: | 2.5h |
| Reactivity: | Human |
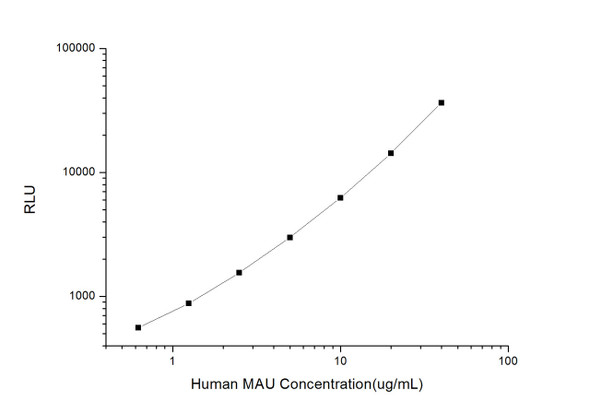
| Detection Range: | 1.56-100 ng/mL |
| Sensitivity: | 0.30 ng/mL |
| Sample Type & Sample Volume: | Urine, 50μL |
| Specificity: | This kit recognizes Human MAU in samples. No significant or interference between Human MAU and analogues was observed. |
| Reproducibility: | Both intra-CV and inter-CV are < 10%. |
| Application: | This ELISA kit applies to the in vitro quantitative determination of Human MAU concentrations in urine.Please consult technical support for the applicability if other biological fluids need to be tested. |
This ELISA kit uses the Sandwich-ELISA principle. The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to Human MAU. Samples (or Standards) and biotinylated detection antibody specific for Human MAU are added to the micro ELISA plate wells. Human MAU would combine with the specific antibody. Then Avidin-Horseradish Peroxidase (HRP) conjugate are added successively to each micro plate well and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human MAU, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 ± 2 nm. The OD value is proportional to the concentration of Human MAU. You can calculate the concentration of Human MAU in the samples by comparing the OD of the samples to the standard curve.
| Kit Components: | An unopened kit can be stored at 2-8℃ for six months. After test, the unused wells and reagents should be stored according to the table below.
|
| 1. | Add 50μL standard or sample to the wells, immediately add 50μL Biotinylated Detection Ab working solution to each well. Incubate for 90 min at 37°C |
| 2. | Aspirate and wash the plate for 3 times |
| 3. | Add 100μL HRP conjugate working solution. Incubate for 30 min at 37°C. Aspirate and wash the plate for 5 times |
| 4. | Add 90μL Substrate Reagent. Incubate for 15 min at 37°C |
| 5. | Add 50μL Stop Solution |
| 6. | Read the plate at 450nm immediately. Calculation of the results |
Microalbuminuria is a subtle increase in the urinary excretion of the protein albumin that cannot be detected by a conventional assay. In diabetes, microalbuminuria is an early sign of diabetic kidney disease. Specifically, the excretion of greater than 30 mg and less than 300 mg a day of albumin in the urine. The normal urinary albumin is less than 30 mg per 24 hours and 300 mg or more of urinary albumin per day is considered gross albuminuria. The phenomenon of albuminuria has been recognized for more than 200 years, and its association with kidney disease dates to the epochal insights of Richard Bright in 1827[1]. Microalbuminuria is caused by glomerular capillary injury and so may be a marker for diffuse endothelial dysfunction. According to Steno hypothesis, albuminuria might reflect a general vascular dysfunction and leakage of albumin and other plasma macromolecules such as low density lipoproteins into the vessel wall that may lead to inflammatory responses and in turn start the atherosclerotic process [2].