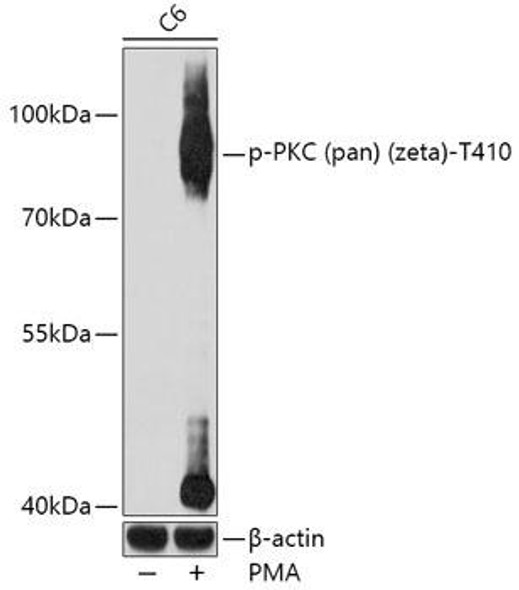
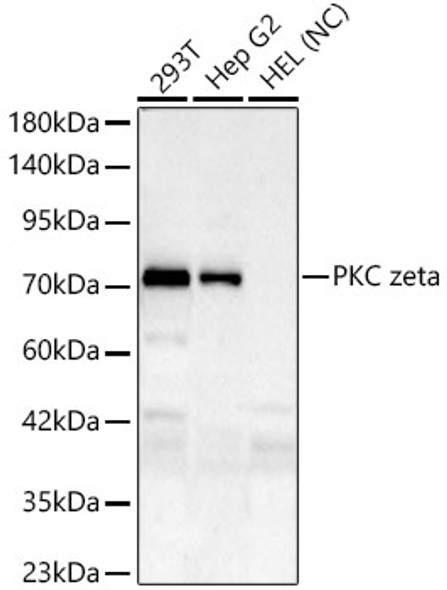
Protein kinase C (PKC) zeta is a member of the PKC family of serine/threonine kinases which are involved in a variety of cellular processes such as proliferation, differentiation and secretion. Unlike the classical PKC isoenzymes which are calcium-dependent, PKC zeta exhibits a kinase activity which is independent of calcium and diacylglycerol but not of phosphatidylserine. Furthermore, it is insensitive to typical PKC inhibitors and cannot be activated by phorbol ester. Unlike the classical PKC isoenzymes, it has only a single zinc finger module. These structural and biochemical properties indicate that the zeta subspecies is related to, but distinct from other isoenzymes of PKC. Alternative splicing results in multiple transcript variants encoding different isoforms.


_WB_01__39721__26504.1706546659.jpg?c=1)