Therapeutic Drug Monitoring
Pembrolizumab (Keytruda®) ELISA Kit
- SKU:
- HUMB00046
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Pembrolizumab (Keytruda®)
- Research Area:
- Checkpoint Inhibitors
Description
Pembrolizumab (Keytruda®) ELISA Kit
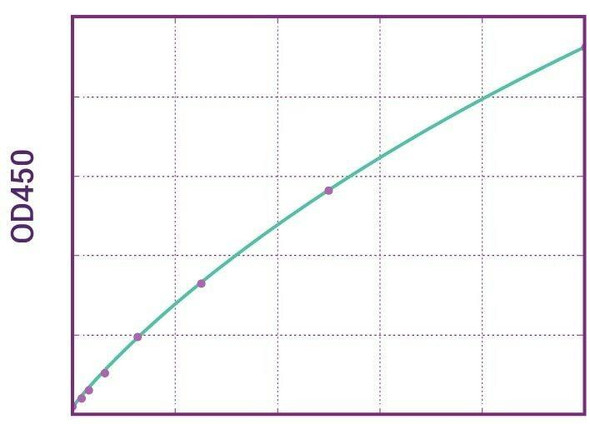
Enzyme-linked immunosorbent assay for the quantitative determination of Pembrolizumab (Keytruda®) in serum and plasma. Assay Genie Pembrolizumab ELISA has been specially developed for the quantitative analysis Pembrolizumab in serum and plasma samples between the Cmin and Cmax range of concentrations indicated in the pharmacokinetics section of the technical manual.
Pembrolizumab (Keytruda®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtitre plate coated with the reactant specific for Pembrolizumab (Keytruda®). Following incubation wells are washed and then horse radish peroxidase (HRP) conjugate is added and binds to Pembrolizumab. After incubation, the wells are washed, and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of Pembrolizumab (Keytruda®) in the sample or standard. Results of samples can be determined directly using the standard curve.
Pembrolizumab (Keytruda®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (μl) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 20 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Keytruda |
Pembrolizumab (Keytruda®) - Key Information
Pembrolizumab (Keytruda®) mode of action
Pembrolizumab (Keytruda®) is a humanized monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. When pembrolizumab (Keytruda®) binds to the PD-1 receptor and blocks both immune-suppressing ligands, PD-L1 and PD-L2, it helps to restore T-cell response and immune response.
Pembrolizumab (Keytruda®) uses
Pembrolizumab (Keytruda®) is used for the treatment of several types of cancer such as, Melanoma, Non-Small Cell Lung Cancer and Head and Neck Cancer. Due to its success in clinical trials, Pembrolizumab was approved early to allow quick patient access and was given breakthrough therapy and orphan drug designation. Pembrolizumab (as Keytruda) was approved by the U.S. Food and Drug Administration to treat advanced cases of the most common type of lung malignancy, non-small cell lung cancer on Oct. 2, 2015.
Keytruda was additionally approved for the treatment of Classical Hodgkin Lymphoma (cHL) in March 2017 Pembrolizumab is a programmed death receptor-1 (PD-1)-blocking antibody indicated for the treatment of the patients with unresectable or metastatic melanoma.
Pembrolizumab (Keytruda®) treatment
Upregulation of PD-1 ligands is a mechanism for tumours to evade antitumor immune response. When PD-1 binds its ligand, the T cell receives an inhibitory signal, which leads to T cell anergy and blockade of anti-tumour immune response. Instead of directly targeting tumor tissue to induce tumor cell death, Pembrolizumab acts as a checkpoint inhibitor to stimulate immune responses to eliminate cancer cells.
Steady-state concentrations of pembrolizumab were reached by 19 weeks of repeated dosing with an every 3- week regimen and the systemic accumulation was 2.2-fold. The peak concentration (Cmax), trough concentration (Cmin), and area under the plasma concentration versus time curve at steady state (AUCss) of pembrolizumab increased dose proportionally in the dose range of 2 to 10 mg/kg every 3 weeks.
Pembrolizumab (Keytruda®) ELISA Kit Contents
| Size | Kit Contents |
1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
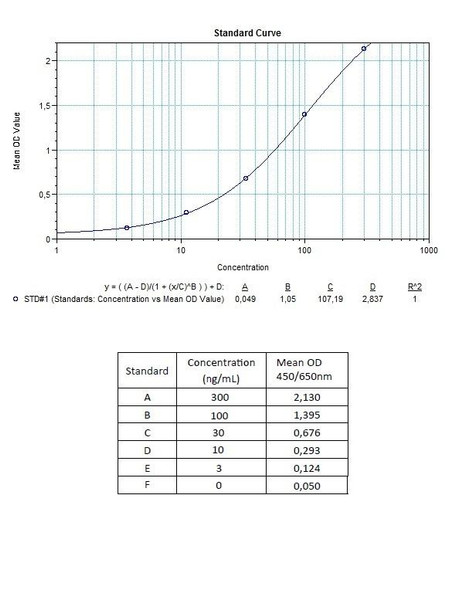
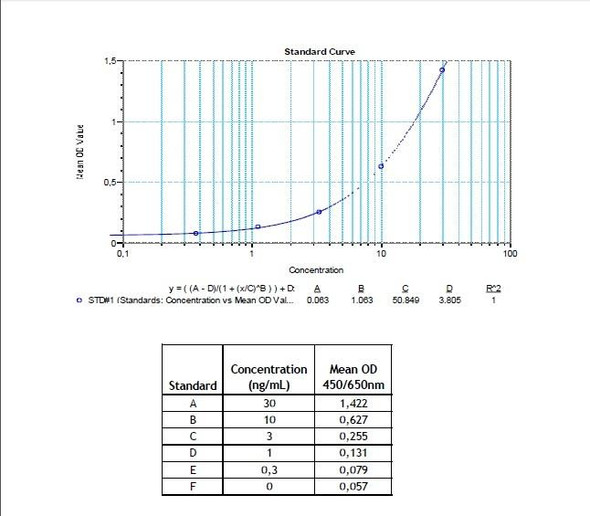
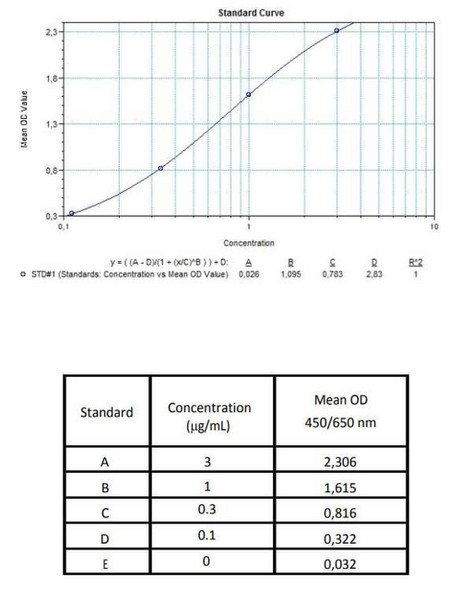
7 x 0.3 mL | Pembrolizumab Standards A-E, High Level Control, Low Level Control 1; 0.3; 0.1; 0.03 and 0 microgram/mL |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Pembrolizumab (Keytruda®) ELISA Kit Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 10 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
3 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
TRADEMARKS
Keytruda® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.