Based on sequence similarity to VEGF-A, a gene encoding a VEGF homologue has recently been discovered in the genome of Orf virus (OV) (Lyttle et al., 1994). Different isolates of Orf virus show significant amino acid sequence similarity to VEGF-A and described as a viral virulence factor that appears to be derived from captured host genes. All eight cysteine residues of the central cysteine knot motif characteristic of members of the VEGF family are conserved among other residues in the VEGF-E proteins (Dehio et al., 1999; Wise et al., 1999). Alignment of all mammalian VEGF sequences indicated that VEGF-E is distinct from the previously described VEGFs but most closely related to VEGF-A. Like VEGF-A, VEGF-E was found to bind with high affinity to VEGF receptor-2 (KDR) resulting in receptor autophosphorylation, whilst in contrast to VEGF-A, VEGF-E can not bind to VEGF receptor-1 (Flt-1). Furthermore VEGF-E can also not bind to VEGF receptor-3 (FLT-4). Therefore VEGF-E is a potent angiog
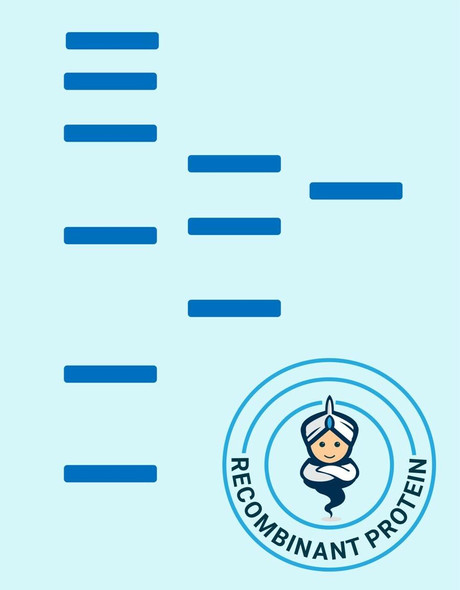
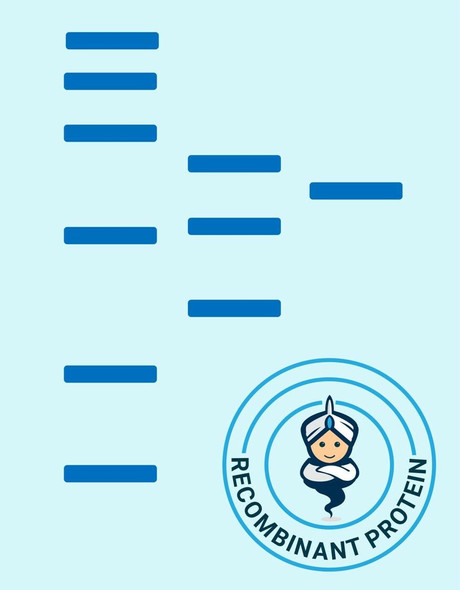
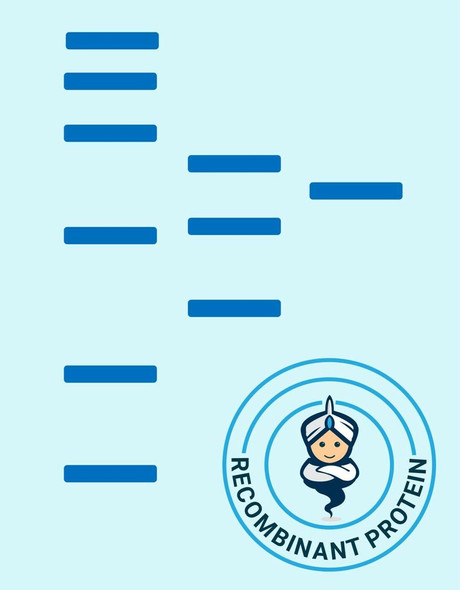
A DNA sequence encoding the mature variant of ovVEGF-E isolate D1701 (Dehio et al., 1999; GenBank accession No. AF106020) was expressed in E. coli as a 132 amino acid residue fusion protein with an N-terminal His-tag sequence and a thrombin cleavage site. Recombinant VEGF-E homodimer was dimerized in vitro and has a predicted mass of approximately 35 kDa.