Therapeutic Drug Monitoring
Natalizumab (Tysabri®)Free drug ELISA Kit
- SKU:
- HUMB00052
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Natalizumab (Tysabri®)
- Research Area:
- Anti-Inflammatory
Description
Natalizumab ELISA has been especially developed for the quantitative analysis of free Natlizumab in serum and plasma samples. Natalizumab ELISA is optimized with Tysabri®.
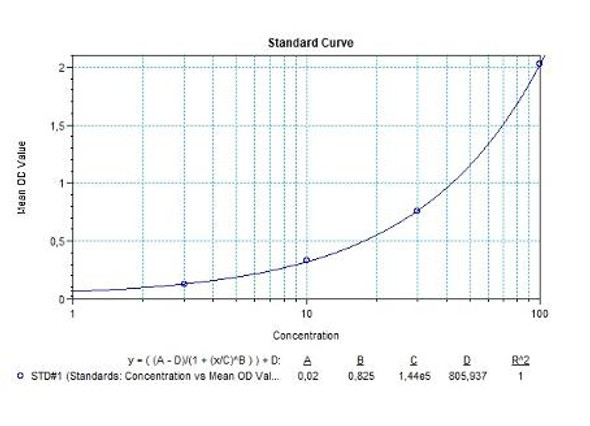
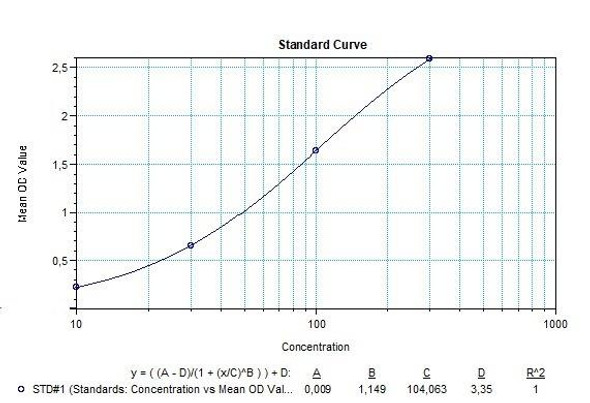
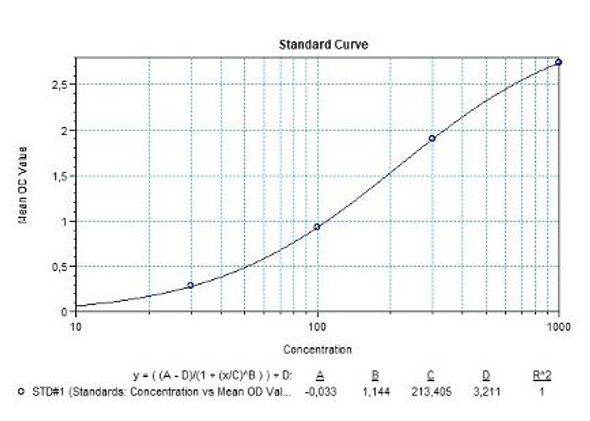
Natalizumab (Tysabri®) Free drug ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the reactant for natalizumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to natalizumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of natalizumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Natalizumab (Tysabri®) Free drug ELISA Product Information
| Information | Description |
| Application | Free Drug |
| Required Volume | 10 µL |
| Total Time | 70 Minutes |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit | 30 ng/mL |
| Spike Recovery | 85-115% |
| Shelf Life (year) | 1 |
| Alternative Names | - |
About Natalizumab (Tysabri®)
Humanized IgG4k monoclonal antibody produced in murine myeloma cells. Natalizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to a4-integrin. Natalizumab was voluntarily withdrawn from U.S. market because of risk of Progressive multifocal leukoencephalopathy (PML).
In multiple sclerosis, lesions are believed to occur when activated inflammatory cells, including T-lymphocytes, cross the blood-brain barrier (BBB). Leukocyte migration across the BBB involves interaction between adhesion molecules on inflammatory cells, and their counter-receptors present on endothelial cells of the vessel wall. The clinical effect of natalizumab in multiple sclerosis may be a secondary result of its blockade of the molecular interaction of a 4b 1-integrin expressed by inflammatory cells with VCAM-1 on vascular endothelial cells, and with CS-1 and/or osteopontin expressed by parenchymal cells in the brain. α4-integrin is required for white blood cells to move into organs, therefore, natalizumab prevents these immune cells from crossing blood vessel walls to reach affected organs thereby decreasing inflamation.
Several randomized controlled trials have demonstrated that natalizumab is effective in increasing rates of remission and 3 maintaining symptom-freestatus in patients with Chron’s disease. Natalizumab may be appropriate in patients who do not respond to medications that block tumor necrosis factoralpha such as infliximab, with some evidance to support combination treatment of Chron’s disesase with natalizumab and infiliximab may be helpful in inducing remission. Treatment of adolescent patients with natalizumab demonstrates an effectiveness similaar to that of adult patients. ELISA Genie kits can be used for drug level and anti-drug antibodies measurements.
Natalizumab ELISA products: