Mouse Cell Biology ELISA Kits 1
Mouse PGF2 alpha (Prostaglandin F2 Alpha) ELISA Kit (MOES01671)
- SKU:
- MOES01671
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Sensitivity:
- 9.38pg/mL
- Range:
- 15.63-1000pg/mL
- ELISA Type:
- Competitive
- Reactivity:
- Mouse
- Sample Type:
- Serum, plasma and other biological fluids
Description
| Assay type: | Competitive-ELISA |
| Format: | 96T |
| Assay time: | 2.5h |
| Reactivity: | Mouse |
| Detection Method: | Colormetric |
| Detection Range: | 15.63-1000 pg/mL |
| Sensitivity: | 9.38 pg/mL |
| Sample Volume: | 50µL |
| Sample Type: | Serum, plasma and other biological fluids |
| Specificity: | This kit recognizes Mouse PGF2 alpha in samples. No significant cross-reactivity or interference between Mouse PGF2 alpha and analogues was observed. |
This ELISA kit uses Competitive-ELISA as the method. The microtiter plate provided in this kit has been pre-coated with Mouse PGF2 alpha. During the reaction, Mouse PGF2 alpha in the sample or standard competes with a fixed amount of Mouse PGF2 alpha on the solid phase supporter for sites on the Biotinylated Detection Ab specific to Mouse PGF2 alpha. Excess conjugate and unbound sample or standard are washed from the plate, and Avidin conjugated to Horseradish Peroxidase (HRP) are added to each microplate well and incubated. Then a TMB substrate solution is added to each well. The enzyme-substrate reaction is terminated by adding Stop Solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of Mouse PGF2 alpha in the samples is then determined by comparing the OD of the samples to the standard curve.
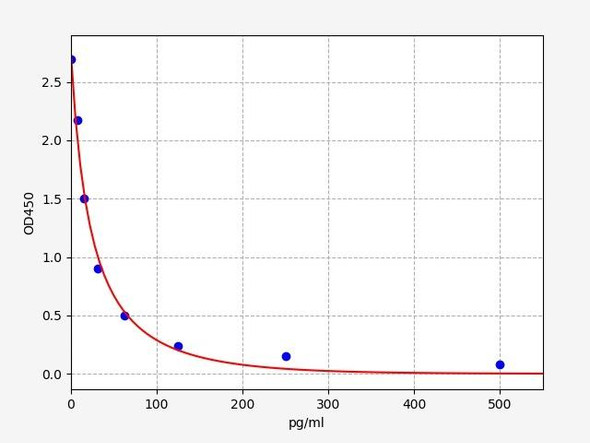
As the OD values of the standard curve may vary according to the conditions of the actual assay performance (e. g. operator, pipetting technique, washing technique or temperature effects), the operator should establish a standard curve for each test. Typical standard curve and data is provided below for reference only.
| Concentration(pg/mL) | O.D | Average |
| 1000 | 0.433 0.477 | 0.455 |
| 500 | 0.562 0.604 | 0.583 |
| 250 | 0.802 0.782 | 0.792 |
| 125 | 1.088 1.092 | 1.09 |
| 62.5 | 1.438 1.434 | 1.436 |
| 31.25 | 1.756 1.754 | 1.755 |
| 15.63 | 1.983 2.001 | 1.992 |
| 0 | 2.319 2.323 | 2.321 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Mouse PGF2 alpha were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Mouse PGF2 alpha were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 47.50 | 147.60 | 486.00 | 49.20 | 144.20 | 512.00 |
| Standard deviation | 2.60 | 6.50 | 15.60 | 3.20 | 7.90 | 20.00 |
| C V (%) | 5.47 | 4.40 | 3.21 | 6.50 | 5.48 | 3.91 |
Recovery
The recovery of Mouse PGF2 alpha spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
| Serum (n=5) | 96-111 | 102 |
| EDTA plasma (n=5) | 93-107 | 98 |
| Cell culture media (n=5) | 95-108 | 100 |
Linearity
Samples were spiked with high concentrations of Mouse PGF2 alpha and diluted with Reference Standard & Sample Diluent to produce samples with values within the range of the assay.
| Serum (n=5) | EDTA plasma (n=5) | Cell culture media (n=5) | ||
| 1:2 | Range (%) | 90-102 | 85-99 | 88-100 |
| Average (%) | 96 | 92 | 95 | |
| 1:4 | Range (%) | 83-97 | 94-105 | 101 |
| Average (%) | 89 | 100 | 101 | |
| 1:8 | Range (%) | 87-101 | 90-104 | 93-105 |
| Average (%) | 92 | 97 | 99 | |
| 1:16 | Range (%) | 83-96 | 86-101 | 97-115 |
| Average (%) | 90 | 92 | 105 |
An unopened kit can be stored at 4°C for 1 month. If the kit is not used within 1 month, store the items separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
| Micro ELISA Plate(Dismountable) | 8 wells ×12 strips | -20°C, 6 months |
| Reference Standard | 2 vials | |
| Concentrated Biotinylated Detection Ab (100×) | 1 vial, 120 µL | |
| Concentrated HRP Conjugate (100×) | 1 vial, 120 µL | -20°C (shading light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 4°C, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent | 1 vial, 10 mL | 4°C (shading light) |
| Stop Solution | 1 vial, 10 mL | 4°C |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
- Set standard, test sample and control (zero) wells on the pre-coated plate and record their positions. It is recommended to measure each standard and sample in duplicate. Note: add all solutions to the bottom of the plate wells while avoiding contact with the well walls. Ensure solutions do not foam when adding to the wells.
- Add 50µL of Standard, Blank or Sample to their respective wells. The blank well is added with Sample / Standard dilution buffer.
- Immediately add 50 µL of Biotin-detection antibody working solution to each well.
- Cover with a plate seal and gently tap the plate to ensure thorough mixing. Incubate for 45minutes at 37°C.
- Aspirate or decant the solution from the plate and add 350µL of wash buffer to each welland incubate for 1-2 minutes at room temperature. Aspirate the solution from each well andclap the plate on absorbent filter paper to dry. Repeat this process 3 times. Note: a microplatewasher can be used in this step and other wash steps.
- Add 100µL of HRP Conjugate working solution to each well and over with a plate seal. Incubate for 30 minutes at 37°C.
- Repeat the aspiration/wash process 5 times according to step 5.
- Add 90µL of the Substrate reagent to each well and cover with a new plate seal. Incubatefor approximately 15 minutes at 37°C and protect from light. The reaction time can beshortened or extended according to the colour change, but not by more than 30 minutes. Whenapparent gradient appears in standard wells, terminate the reaction.
- Stop: Add 50µL of Stop Solution to each well (wells will develop a yellow color immediately). Note: Adding the stop solution should be done in the same order as the substrate solution.
- Determine the optical density (OD value) of each well immediately with a microplate readerset at 450 nm. In advance, preheat the instrument and set the testing parameters.