Mouse Immunology ELISA Kits
Mouse C5 (Complement Component 5) ELISA Kit (MOES00896)
- SKU:
- MOES00896
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- P06684
- Sensitivity:
- 0.09ng/mL
- Range:
- 0.16-10ng/mL
- ELISA Type:
- Sandwich
- Synonyms:
- CPAMD4
- Reactivity:
- Mouse
- Sample Type:
- Serum, plasma and other biological fluids
- Research Area:
- Immunology
Description
| Assay type: | Sandwich |
| Format: | 96T |
| Assay time: | 4.5h |
| Reactivity: | Mouse |
| Detection Method: | Colormetric |
| Detection Range: | 0.16-10 ng/mL |
| Sensitivity: | 0.10 ng/mL |
| Sample Volume Required Per Well: | 100µL |
| Sample Type: | Serum, plasma and other biological fluids |
| Specificity: | This kit recognizes Mouse C5 in samples. No significant cross-reactivity or interference between Mouse C5 and analogues was observed. |
This ELISA kit uses Sandwich-ELISA as the method. The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to Mouse C5. Standards or samples are added to the appropriate micro ELISA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for Mouse C5 and Avidin-Horseradish Peroxidase (HRP) conjugate are added to each micro plate well successively and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Mouse C5, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by adding Stop Solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The OD value is proportional to the concentration of Mouse C5. The concentration of Mouse C5 in samples can be calculated by comparing the OD of the samples to the standard curve.
| UniProt Protein Function: | C5: Activation of C5 by a C5 convertase initiates the spontaneous assembly of the late complement components, C5-C9, into the membrane attack complex. C5b has a transient binding site for C6. The C5b-C6 complex is the foundation upon which the lytic complex is assembled. Defects in C5 are the cause of complement component 5 deficiency (C5D). A rare defect of the complement classical pathway associated with susceptibility to severe recurrent infections, predominantly by Neisseria gonorrhoeae or Neisseria meningitidis. An association study of C5 haplotypes and genotypes in individuals with chronic hepatitis C virus infection shows that individuals homozygous for the C5_1 haplotype have a significantly higher stage of liver fibrosis than individuals carrying at least 1 other allele (PubMed:15995705). |
| UniProt Protein Details: | Protein type:Secreted; Secreted, signal peptide Cellular Component: membrane attack complex; extracellular space; extracellular region Molecular Function:endopeptidase inhibitor activity Biological Process: positive regulation of angiogenesis; in utero embryonic development; immune system process; cytolysis; complement activation, alternative pathway; innate immune response; inflammatory response; complement activation, classical pathway |
| UniProt Code: | P06684 |
| NCBI GenInfo Identifier: | 116608 |
| NCBI Gene ID: | 15139 |
| NCBI Accession: | P06684. 2 |
| UniProt Secondary Accession: | P06684,A2AS36, |
| UniProt Related Accession: | P06684 |
| Molecular Weight: | 188,878 Da |
| NCBI Full Name: | Complement C5 |
| NCBI Synonym Full Names: | hemolytic complement |
| NCBI Official Symbol: | Hc |
| NCBI Official Synonym Symbols: | C5; He; C5a |
| NCBI Protein Information: | complement C5 |
| UniProt Protein Name: | Complement C5 |
| UniProt Synonym Protein Names: | Hemolytic complementCleaved into the following 4 chains:Complement C5 beta chain; Complement C5 alpha chain; C5a anaphylatoxin; Complement C5 alpha' chain |
| Protein Family: | Complement C5 |
| UniProt Gene Name: | C5 |
| UniProt Entry Name: | CO5_MOUSE |
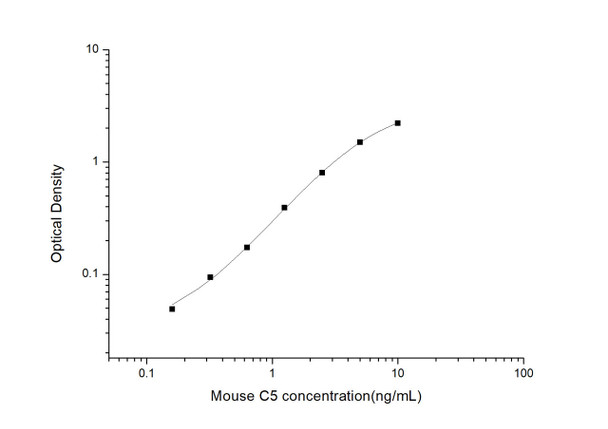
As the OD values of the standard curve may vary according to the conditions of the actual assay performance (e. g. operator, pipetting technique, washing technique or temperature effects), the operator should establish a standard curve for each test. Typical standard curve and data is provided below for reference only.
| Concentration (ng/mL) | O.D | Average | Corrected |
| 10 | 2.285 2.301 | 2.293 | 2.219 |
| 5 | 1.548 1.6 | 1.574 | 1.5 |
| 2.5 | 0.89 0.864 | 0.877 | 0.803 |
| 1.25 | 0.45 0.48 | 0.465 | 0.391 |
| 0.63 | 0.254 0.24 | 0.247 | 0.173 |
| 0.32 | 0.169 0.167 | 0.168 | 0.094 |
| 0.16 | 0.119 0.127 | 0.123 | 0.049 |
| 0 | 0.065 0.083 | 0.074 | -- |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Mouse C5 were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Mouse C5 were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (ng/mL) | 0.47 | 1.19 | 3.99 | 0.45 | 1.07 | 3.63 |
| Standard deviation | 0.02 | 0.06 | 0.20 | 0.02 | 0.06 | 0.15 |
| C V (%) | 4.26 | 5.04 | 5.01 | 4.44 | 5.61 | 4.13 |
Recovery
The recovery of Mouse C5 spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
| Serum (n=5) | 84-100 | 91 |
| EDTA plasma (n=5) | 94-109 | 101 |
| Cell culture media (n=5) | 91-103 | 97 |
Linearity
Samples were spiked with high concentrations of Mouse C5 and diluted with Reference Standard & Sample Diluent to produce samples with values within the range of the assay.
| Serum (n=5) | EDTA plasma (n=5) | Cell culture media (n=5) | ||
| 1:2 | Range (%) | 96-109 | 87-101 | 97-111 |
| Average (%) | 101 | 93 | 103 | |
| 1:4 | Range (%) | 89-101 | 81-95 | 87-101 |
| Average (%) | 96 | 88 | 93 | |
| 1:8 | Range (%) | 87-100 | 88-100 | 85-95 |
| Average (%) | 93 | 93 | 90 | |
| 1:16 | Range (%) | 94-109 | 82-92 | 81-95 |
| Average (%) | 99 | 87 | 87 |
An unopened kit can be stored at 4°C for 1 month. If the kit is not used within 1 month, store the items separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
| Micro ELISA Plate(Dismountable) | 8 wells ×12 strips | -20°C, 6 months |
| Reference Standard | 2 vials | |
| Concentrated Biotinylated Detection Ab (100×) | 1 vial, 120 µL | |
| Concentrated HRP Conjugate (100×) | 1 vial, 120 µL | -20°C(shading light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 4°C, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent | 1 vial, 10 mL | 4°C(shading light) |
| Stop Solution | 1 vial, 10 mL | 4°C |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
- Set standard, test sample and control (zero) wells on the pre-coated plate and record theirpositions. It is recommended to measure each standard and sample in duplicate. Note: addall solutions to the bottom of the plate wells while avoiding contact with the well walls. Ensuresolutions do not foam when adding to the wells.
- Aliquot 100µl of standard solutions into the standard wells.
- Add 100µl of Sample / Standard dilution buffer into the control (zero) well.
- Add 100µl of properly diluted sample (serum, plasma, tissue homogenates and otherbiological fluids) into test sample wells.
- Cover the plate with the sealer provided in the kit and incubate for 90 min at 37°C.
- Aspirate the liquid from each well, do not wash. Immediately add 100µL of BiotinylatedDetection Ab working solution to each well. Cover the plate with a plate seal and gently mix. Incubate for 1 hour at 37°C.
- Aspirate or decant the solution from the plate and add 350µL of wash buffer to each welland incubate for 1-2 minutes at room temperature. Aspirate the solution from each well andclap the plate on absorbent filter paper to dry. Repeat this process 3 times. Note: a microplatewasher can be used in this step and other wash steps.
- Add 100µL of HRP Conjugate working solution to each well. Cover with a plate seal andincubate for 30 min at 37°C.
- Aspirate or decant the solution from each well. Repeat the wash process for five times asconducted in step 7.
- Add 90µL of Substrate Reagent to each well. Cover with a new plate seal and incubate forapproximately 15 min at 37°C. Protect the plate from light. Note: the reaction time can beshortened or extended according to the actual color change, but not by more than 30min.
- Add 50 µL of Stop Solution to each well. Note: Adding the stop solution should be done inthe same order as the substrate solution.
- Determine the optical density (OD value) of each well immediately with a microplate readerset at 450 nm.