Therapeutic Drug Monitoring
Ipilimumab (Yervoy®) ELISA Kit
- SKU:
- HUMB00048
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Ipilimumab (Yervoy®)
- Research Area:
- Checkpoint Inhibitors
Description
Ipilimumab (Yervoy®) ELISA Kit
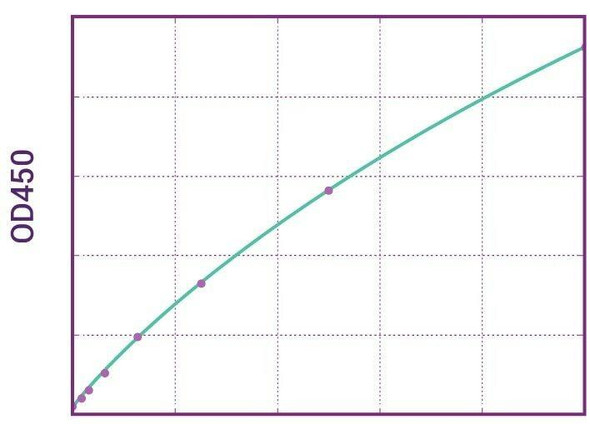
Enzyme-linked immunosorbent assay for the quantitative determination of specific Ipilimumab (Yervoy®) in human serum and plasma. The Assay Genie Ipilimumab (Yervoy®) ELISA has been especially developed for the quantitative analysis of free Ipilimumab in serum and plasma samples.
Ipilimumab (Yervoy®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtitre plate coated with the reactant specific for ipilimumab (Yervoy®). Following incubation wells are washed and then horse radish peroxidase (HRP) is added and binds to ipilimumab. After incubation, the wells are washed, and the bound enzymatic activity is detected by addition of chromogensubstrate. The colour developed is proportional to the amount of ipilimumab (Yervoy®) in the sample or standard. Results of samples can be determined directly using the standard curve.
Ipilimumab (Yervoy®) ELISA Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 70 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 100 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-CTLA-4 mAb Yervoy |
Ipilimumab (Yervoy®) - Key Information
Ipilimumab (Yervoy®) mode of action
Ipilimumab, a recombinant human monoclonal antibody (IgG1 kappa immunoglobin), is an antineoplastic agent. Ipilimumab is a fully human IgG1κ antibody that binds to CTLA-4 (cytotoxic T lymphocyte associated antigen 4), a molecule on T-cells that is indicated for unresectable or metastatic melanoma. The absence or presence of CTLA-4 can augment or suppress the immune system's T-cell response in fighting disease. Ipilimumab is designed to block the activity of CTLA-4, thereby sustaining an active immune response in its attack on cancer cells. The proposed mechanism of action is indirect and may be through T-cell - mediated anti-tumor immune responses.
Ipilimumab (Yervoy®) uses
Ipilimumab is indicated for the treatment of unresectable or metastatic melanoma in adults. It is also used to reduce the risk of the deadly skin cancer returning after surgery. Ipilimumab (Yervoy) shrinks tumors and contributes to patient recovery with advanced melanoma live longer. It is also approved for adjuvant therapy.
Ipilimumab (Yervoy®) pharmacokinetics and pharmacodynamics
In one pharmacokinetic study of patients with unresectable or metastatic melanoma peak concentrations, trough concentrations, and area under the curve (AUC) were found to be dose proportional in the dosage range examined (0.3, 3, or 10mg/kg every 3 weeks for four doses). The metabolism of ipilimumab does not involve the cytochrome P450 enzyme system. Because ipilimumab is a protein it is expected to be degraded into small peptides and amino acids by proteolytic enzymes. Clearance was measured to be 15.3mL/hr-16.8 mL/hr. In one pharmacokinetic study examining ipilimumab administered every 3 weeks, clearance was found to be time invariant.
Minimal systemic accumulation was observed (accumulation index of 1.5-fold or less). Steady state concentrations was reached by the third dose. Clearance will increase with increasing body weight; however, no dose adjustment is needed if administration occurs on a mg/kg basis. The following had no clinically meaningful influence on clearance: Age (range 26-86 years), gender, creatinine clearance, baseline AST, total bilirubin, ALT levels, concomitant use of budesonide, performance status, HLA-A2*0201 status, positive anti-ipilimumab antibody status, prior use of systemic anticancer therapy, baseline lactate dehydrogenase levels.
The pharmacodynamics of Ipilimumab are not completely understood. In melanoma patients receiving Ipilimumab, the mean peripheral blood absolute lymphocyte counts (ALC) increased throughout the induction dosing period. This increase occurred in a dose-dependent fashion in Phase 2 studies. Ipilimumab given with or without gp100 at 3 mg/kg increased ALC throughout the induction dosing period, but no meaningful change in ALC occurred in the control group who received an investigational peptide vaccine alone. Furthermore, ipilimumab binds to CTLA-4 with high affinity (Kd = 5.24 ± 3.62 nM). As a result, ligands CD80 and CD86 are blocked from binding to CTLA-4 with a minimum EC50 value of 0.2 μg/mL.
Ipilimumab (Yervoy®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
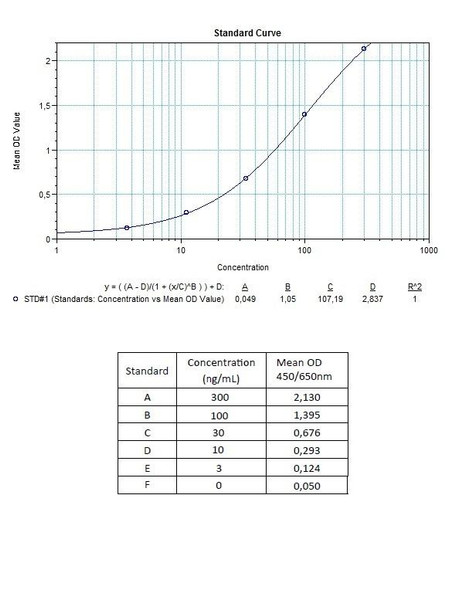
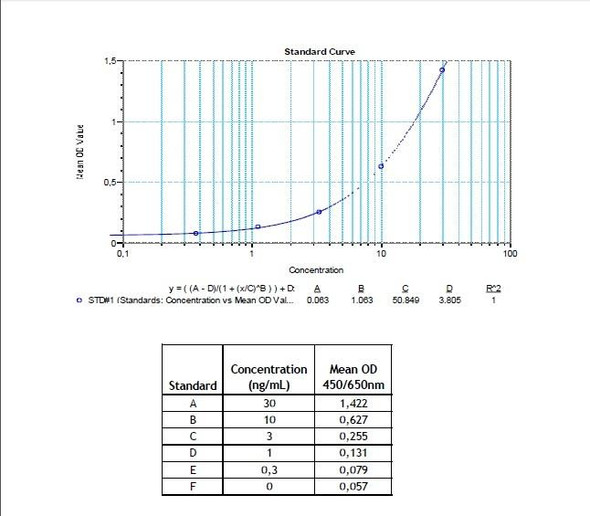
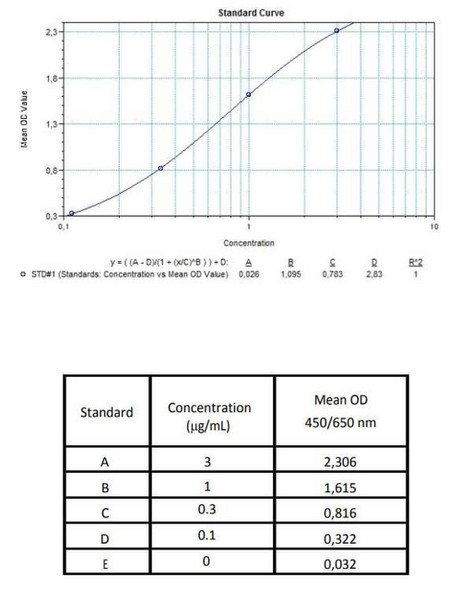
7 x 0.3 mL | Ipilimumab Standards A-E, High Level Control, Low Level Control 3000, 1000, 300, 100 and 0 ng/mL. Ready to use. Used for construction |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Ipilimumab (Yervoy®) ELISA Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 10 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. |
3 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
YERVOY is a trademark of Bristol-Myers Squibb Company.