Human Cell Biology ELISA Kits 5
Human TBG (Thyroxine Binding Globulin) CLIA Kit (HUES00844)
- SKU:
- HUES00844
- Product Type:
- ELISA Kit
- ELISA Type:
- CLIA Kit
- Size:
- 96 Assays
- Sensitivity:
- 0.09ng/mL
- Range:
- 0.16-10ng/mL
- ELISA Type:
- Sandwich
- Reactivity:
- Human
- Sample Type:
- Serum, plasma and other biological fluids
- Research Area:
- Cell Biology
Description
| Assay type: | Sandwich |
| Format: | 96T |
| Assay time: | 4.5h |
| Reactivity: | Human |
| Detection method: | Chemiluminescence |
| Detection range: | 0.16-10 ng/mL |
| Sensitivity: | 0.09 ng/mL |
| Sample volume: | 100µL |
| Sample type: | Serum, plasma and other biological fluids |
| Repeatability: | CV < 15% |
| Specificity: | This kit recognizes Human TBG in samples. No significant cross-reactivity or interference between Human TBG and analogues was observed. |
This kit uses Sandwich-CLIA as the method. The micro CLIA plate provided in this kit has been pre-coated with an antibody specific to Human TBG. Standards or samples are added to the appropriate micro CLIA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for Human TBG and Avidin-Horseradish Peroxidase (HRP) conjugate are added to each micro plate well successively and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human TBG, biotinylated detection antibody and Avidin-HRP conjugate will appear fluorescence. The Relative light unit (RLU) value is measured spectrophotometrically by the Chemiluminescence immunoassay analyzer. The RLU value is positively associated with the concentration of Human TBG. The concentration of Human TBG in the samples can be calculated by comparing the RLU of the samples to the standard curve.
| UniProt Protein Function: | SERPINA7: Major thyroid hormone transport protein in serum. Defects in SERPINA7 are a cause of thyroxine-binding globulin deficiency (TBG deficiency). Mutations in the SERPINA7 gene can result as a whole spectrum of deficiencies, characterized by either reduced or increased TBG levels in the serum. Patients show, respectively, reduced or elevated protein- bound iodine but are euthyroid. Belongs to the serpin family. |
| UniProt Protein Details: | Protein type:Secreted, signal peptide; Secreted Chromosomal Location of Human Ortholog: Xq22. 2 Molecular Function:serine-type endopeptidase inhibitor activity |
| NCBI Summary: | There are three proteins including thyroxine-binding globulin (TBG), transthyretin and albumin responsible for carrying the thyroid hormones thyroxine (T4) and 3,5,3'-triiodothyronine (T3) in the bloodstream. This gene encodes the major thyroid hormone transport protein, TBG, in serum. It belongs to the serpin family in genomics, but the protein has no inhibitory function like many other members of the serpin family. Mutations in this gene result in TGB deficiency, which has been classified as partial deficiency, complete deficiency, and excess, based on the level of serum TBG. Alternatively spliced transcript variants encoding different isoforms have been found, but the full-length nature of these variants has not been determined. [provided by RefSeq, Jun 2012] |
| UniProt Code: | P05543 |
| NCBI GenInfo Identifier: | 1351236 |
| NCBI Gene ID: | 6906 |
| NCBI Accession: | P05543. 2 |
| UniProt Secondary Accession: | P05543,D3DUX1, |
| UniProt Related Accession: | P05543 |
| Molecular Weight: | 46,325 Da |
| NCBI Full Name: | Thyroxine-binding globulin |
| NCBI Synonym Full Names: | serpin family A member 7 |
| NCBI Official Symbol: | SERPINA7 |
| NCBI Official Synonym Symbols: | TBG |
| NCBI Protein Information: | thyroxine-binding globulin |
| UniProt Protein Name: | Thyroxine-binding globulin |
| UniProt Synonym Protein Names: | Serpin A7; T4-binding globulin |
| Protein Family: | Thyroxine-binding globulin |
| UniProt Gene Name: | SERPINA7 |
| UniProt Entry Name: | THBG_HUMAN |
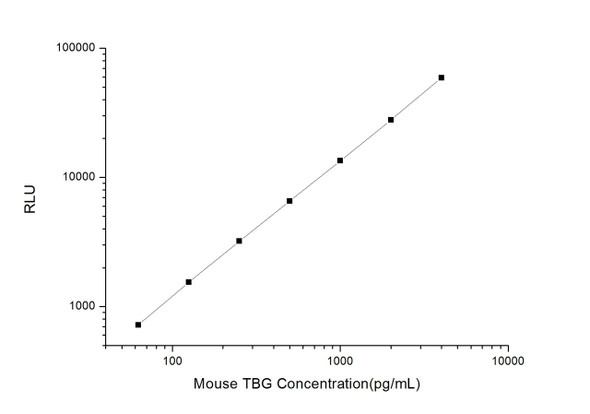
As the RLU values of the standard curve may vary according to the conditions of the actual assay performance (e. g. operator, pipetting technique, washing technique or temperature effects), the operator should establish a standard curve for each test. Typical standard curve and data is provided below for reference only.
| Concentration (ng/mL) | RLU | Average | Corrected |
| 10 | 32321 32621 | 32471 | 32447 |
| 5 | 14435 16505 | 15470 | 15446 |
| 2.5 | 7724 7282 | 7503 | 7479 |
| 1.25 | 3392 3914 | 3653 | 3629 |
| 0.63 | 1831 1691 | 1761 | 1737 |
| 0.31 | 851 797 | 824 | 800 |
| 0.16 | 333 381 | 357 | 333 |
| 0 | 23 25 | 24 | -- |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Human TBG were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Human TBG were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (ng/mL) | 0.46 | 1.19 | 3.77 | 0.47 | 1.26 | 3.63 |
| Standard deviation | 0.05 | 0.11 | 0.27 | 0.06 | 0.12 | 0.30 |
| C V (%) | 10.87 | 9.24 | 7.16 | 12.77 | 9.52 | 8.26 |
Recovery
The recovery of Human TBG spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
| Serum (n=5) | 100-111 | 105 |
| EDTA plasma (n=5) | 89-99 | 94 |
| Cell culture media (n=5) | 86-99 | 91 |
Linearity
Samples were spiked with high concentrations of Human TBG and diluted with Reference Standard & Sample Diluent to produce samples with values within the range of the assay.
| Serum (n=5) | EDTA plasma (n=5) | Cell culture media (n=5) | ||
| 1:2 | Range (%) | 88-101 | 101-115 | 83-96 |
| Average (%) | 95 | 107 | 90 | |
| 1:4 | Range (%) | 100-113 | 86-96 | 88-102 |
| Average (%) | 107 | 91 | 93 | |
| 1:8 | Range (%) | 93-106 | 86-101 | 93-105 |
| Average (%) | 98 | 93 | 99 | |
| 1:16 | Range (%) | 88-102 | 86-97 | 94-109 |
| Average (%) | 94 | 91 | 100 |
An unopened kit can be stored at 4°C for 1 month. If the kit is not used within 1 month, store the items separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
| Micro CLIA Plate(Dismountable) | 8 wells ×12 strips | -20°C, 6 months |
| Reference Standard | 2 vials | |
| Concentrated Biotinylated Detection Ab (100×) | 1 vial, 120 µL | |
| Concentrated HRP Conjugate (100×) | 1 vial, 120 µL | -20°C(shading light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 4°C, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent A | 1 vial, 5 mL | 4°C (shading light) |
| Substrate Reagent B | 1 vial, 5 mL | 4°C (shading light) |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
- Set standard, test sample and control (zero) wells on the pre-coated plate and record theirpositions. It is recommended to measure each standard and sample in duplicate. Note: addall solutions to the bottom of the plate wells while avoiding contact with the well walls. Ensuresolutions do not foam when adding to the wells.
- Aliquot 100µl of standard solutions into the standard wells.
- Add 100µl of Sample / Standard dilution buffer into the control (zero) well.
- Add 100µl of properly diluted sample (serum, plasma, tissue homogenates and otherbiological fluids. ) into test sample wells.
- Cover the plate with the sealer provided in the kit and incubate for 90 min at 37°C.
- Aspirate the liquid from each well, do not wash. Immediately add 100µL of BiotinylatedDetection Ab working solution to each well. Cover the plate with a plate seal and gently mix. Incubate for 1 hour at 37°C.
- Aspirate or decant the solution from the plate and add 350µL of wash buffer to each welland incubate for 1-2 minutes at room temperature. Aspirate the solution from each well andclap the plate on absorbent filter paper to dry. Repeat this process 3 times. Note: a microplatewasher can be used in this step and other wash steps.
- Add 100µL of HRP Conjugate working solution to each well. Cover with a plate seal andincubate for 30 min at 37°C.
- Aspirate or decant the solution from each well. Repeat the wash process for five times asconducted in step 7.
- Add 100µL of Substrate mixture solution to each well. Cover with a new plate seal andincubate for no more than 5 min at 37°C. Protect the plate from light.
- Determine the RLU value of each well immediately.