Description
| Product Name: | Human MMP-9/CLG4B Recombinant Protein (His tag) |
| Product Code: | RPES5543 |
| Size: | 20µg |
| Species: | Human |
| Expression Host: | E.coli |
| Synonyms: | Matrix metalloproteinase-9, 92 kDa gelatinase, 92 kDa type IV collagenase, Gelatinase B, MMP9, CLG4B, GELB, MANDP2 |
| Mol Mass: | 30.58 kDa |
| AP Mol Mass: | 35 kDa |
| Tag: | N-His |
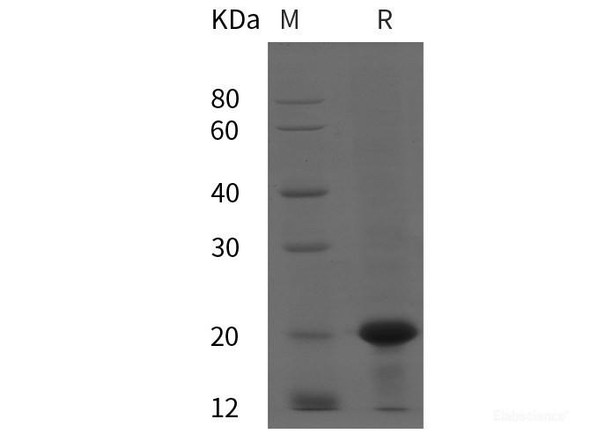
| Purity: | > 95 % as determined by reducing SDS-PAGE. |
| Endotoxin Level: | Please contact us for more information. |
| Bio Activity: | Testing in progress |
| Sequence: | Arg 162-Arg 440 |
| Accession: | P14780 |
| Storage: | Generally, lyophilized proteins are stable for up to 12 months when stored at -20 to -80°C. Reconstituted protein solution can be stored at 4-8°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Shipping: | This product is provided as lyophilized powder which is shipped with ice packs. |
| Formulation: | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Please refer to the specific buffer information in the printed manual. |
| Reconstitution: | Please refer to the printed manual for detailed information. |
| Background: | Matrix metalloproteinases (MMPs) are neutral proteinases that are involved in the breakdown and remodeling of the extracellular matrix (ECM) under a variety of physiological and pathological conditions, such as morphogenesis, differentiation, angiogenesis and tissue remodeling, as well as pathological processes including inflammation, arthritis, cardiovascular diseases, pulmonary diseases and tumor invasion. MMP9, also known as 92-kDa gelatinase B/type IV collagenase, is secreted from neutrophils, macrophages, and a number of transformed cells, and is the most complex family member in terms of domain structure and regulation of its activity. It plays an important role in tissue remodelling in normal and pathological inflammatory processes. MMP-9 is a major secretion product of macrophages and a component of cytoplasmic granules of neutrophils, and is particularly important in the pathogenesis of inflammatory, infectious, and neoplastic diseases in many organs including the lung. This enzyme is also secreted by lymphocytes and stromal cells upon stimulation by inflammatory cytokines, or upon delivery of bi-directional activation signals following integrin-mediated cell-cell or cell-extracellular matrix (ECM) contacts. The dramatic overexpression of MMP-9 in cancer and various inflammatory conditions clearly points to the molecular mechanisms controlling its expression as a potential target for eventual rational therapeutic intervention. |