Description
| Product Name: | Human IFN beta 1a Recombinant Protein (N-His) (active) |
| Product Code: | RPES6450 |
| Size: | 20µg |
| Species: | Human |
| Expression Host: | E.coli |
| Synonyms: | IFNB1, Type I Interferon |
| Mol Mass: | 23.12 kDa |
| Tag: | N-His |
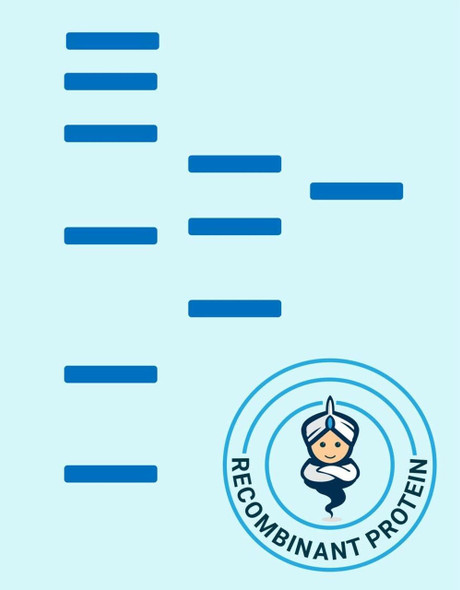
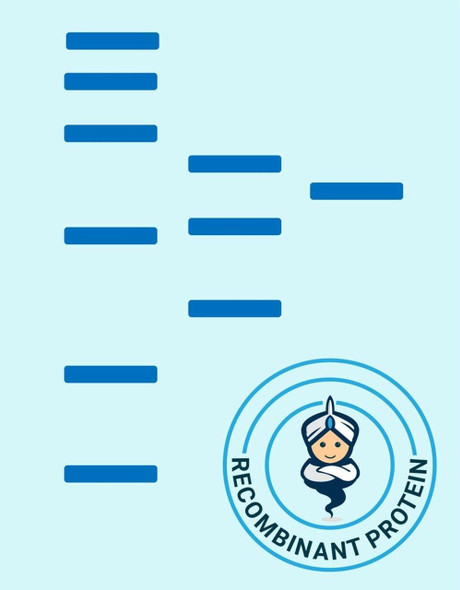
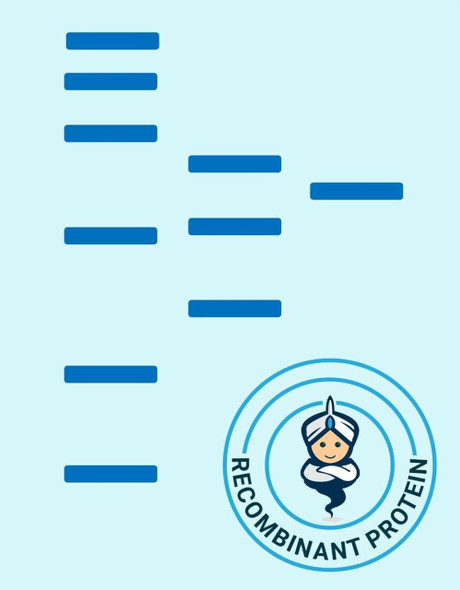
| Purity: | > 98 % as determined by reducing SDS-PAGE. |
| Endotoxin Level: | < 0.1 EU per μg of the protein as determined by the LAL method. |
| Bio Activity: | 1. Measure by its ability to induce apoptosis in HeLa cells. The ED50 for this effect is < 15 ng/mL. 2. Measure by its ability to induce cytotoxicity in TF-1 cells. The ED50 for this effect is < 0.1 ng/mL. The specific activity of recombinant human IFN beta 1a is approximately > 1 x107 IU/ mg. |
| Sequence: | MTNKCLLQIALLLCFSTTALSMSYNLLGFLQRSSNFQCQKLLWQLNGRLEYCLKDRMNFDIPEEIKQLQQFQKEDAALTIYEMLQNIFAIFRQDSSSTGWNETIVENLLANVYHQINHLKTVLEEKLEKEDFTRGKLMSSLHLKRYYGRILHYLKAKEYSHCAWTIVRVEILRNFYFINRLTGYLRN |
| Accession: | P01574 |
| Storage: | Generally, lyophilized proteins are stable for up to 12 months when stored at -20 to -80°C. Reconstituted protein solution can be stored at 4-8°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Shipping: | This product is provided as lyophilized powder which is shipped with ice packs. |
| Formulation: | Lyophilized from sterile PBS, pH 8.0 Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Please refer to the specific buffer information in the printed manual. |
| Reconstitution: | Please refer to the printed manual for detailed information. |
| Background: | Interferons (IFNs) are natural glycoproteins belonging to the cytokine superfamily and are produced by the cells of the immune system of most vertebrates in response to challenges by foreign agents such as viruses, parasites, and tumor cells. Interferon-beta (IFN beta) is an extracellular protein mediator of host defense and homeostasis. IFN beta has well-established direct antiviral, antiproliferative, and immunomodulatory properties. Recombinant IFN beta is approved for the treatment of relapsing-remitting multiple sclerosis. The recombinant IFN beta protein has the theoretical potential to either treat or causes autoimmune neuromuscular disorders by altering the complicated and delicate balances within the immune system networks. It is the most widely prescribed disease-modifying therapy for multiple sclerosis (MS). IFN beta is effective in reducing relapses in secondary progressive MS and may have a modest effect in slowing disability progression. In addition to the common antiviral activity, IFN beta also induces increased production of the p53 gene product which promotes apoptosis and thus has a therapeutic effect against certain cancers. The role of IFN-beta in bone metabolism could warrant its systematic evaluation as a potential adjunct to therapeutic regimens of osteolytic diseases. Furthermore, IFN beta might play a beneficial role in the development of chronic progressive CNS inflammation. |