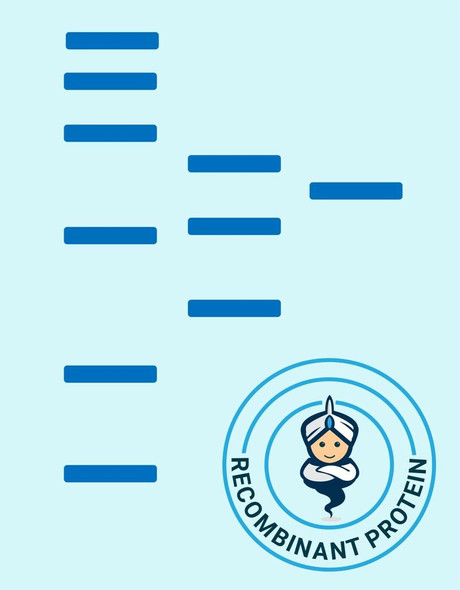
Description
| Product Name: | Human HSPA5 Recombinant Protein |
| Product Code: | RPPB5244 |
| Size: | 20µg |
| Species: | Human |
| Target: | HSPA5 |
| Synonyms: | 78 kDa glucose-regulated protein, GRP-78, Endoplasmic reticulum lumenal Ca(2+)-binding protein grp78, Heat shock 70 kDa protein 5, Immunoglobulin heavy chain-binding protein, BiP, HSPA5, GRP78, MIF2, FLJ26106. |
| Source: | Hi-5 cells |
| Physical Appearance: | Sterile filtered colorless solution. |
| Formulation: | The HSPA5 protein solution (1mg/ml) 20mM Tris-HCl buffer (pH8.0), 10% glycerol, 2mM DTT and 200mM NaCl. |
| Stability: | Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of time.For long term storage it is recommended to add a carrier protein (0.1% HSA or BSA).Avoid multiple freeze-thaw cycles. |
| Purity: | Greater than 90.0% as determined by SDS-PAGE. |
| Amino Acid Sequence: | MEEDKKEDVG TVVGIDLGTT YSCVGVFKNG RVEIIANDQG NRITPSYVAF TPEGERLIGD AAKNQLTSNP ENTVFDAKRL IGRTWNDPSV QQDIKFLPFK VVEKKTKPYI QVDIGGGQTK TFAPEEISAM VLTKMKETAE AYLGKKVTHA VVTVPAYFND AQRQATKDAG TIAGLNVMRI INEPTAAAIA YGLDKREGEK NILVFDLGGG TFDVSLLTID NGVFEVVATN GDTHLGGEDF DQRVMEHFIK LYKKKTGKDV RKDNRAVQKL RREVEKAKRA LSSQHQARIE IESFYEGEDF SETLTRAKFE ELNMDLFRST MKPVQKVLED SDLKKSDIDE IVLVGGSTRI PKIQQLVKEF FNGKEPSRGI NPDEAVAYGA AVQAGVLSGD QDTGDLVLLD VCPLTLGIET VGGVMTKLIP RNTVVPTKKS QIFSTASDNQ PTVTIKVYEG ERPLTKDNHL LGTFDLTGIP PAPRGVPQIE VTFEIDVNGI LRVTAEDKGT GNKNKITITN DQNRLTPEEI ERMVNDAEKF AEEDKKLKER IDTRNELESY AYSLKNQIGD KEKLGGKLSS EDKETMEKAV EEKIEWLESH QDADIEDFKA KKKELEEIVQ PIISKLYGSA GPPPTGEEDT AELEHHHHHH |
Binding immunoglobulin protein (BiP or HSPA5) is a member of the family of ~70kDa heat shock proteins (HSP 70). HSPA5 is a stress response protein which is induced by agents or conditions that adversely affect endoplasmic reticulum (ER) function. HSPA5 is crucial for the proper glycosylation, folding as well as for the maintenance of cell homeostasis and the prevention of apoptosis.
HSPA5 produced in Hi-5 cells is a single, glycosylated polypeptide chain containing 640 amino acids (20-650 a.a.) and having a molecular mass of 71kDa. HSPA5 is fused to an 8 amino acid His Tag at C-Terminus and purified by proprietary chromatographic techniques.
| UniProt Protein Function: | GRP78: a member of the HSP family of molecular chaperones required for endoplasmic reticulum integrity and stress-induced autophagy. Plays a central role in regulating the unfolded protein response (UPR), and is an obligatory component of autophagy in mammalian cells. May play an important role in cellular adaptation and oncogenic survival. One of the client proteins of GRP78 is protein double-stranded RNA-activated protein-like endoplasmic reticulum kinase (PERK). Probably plays a role in facilitating the assembly of multimeric protein complexes inside the ER. |
| UniProt Protein Details: | Protein type:Chaperone; Heat shock protein Chromosomal Location of Human Ortholog: 9q33.3 Cellular Component: signalosome; endoplasmic reticulum membrane; focal adhesion; smooth endoplasmic reticulum; cell surface; mitochondrion; endoplasmic reticulum; endoplasmic reticulum lumen; ER-Golgi intermediate compartment; membrane; melanosome; plasma membrane; integral to endoplasmic reticulum membrane; midbody; nucleus Molecular Function:protein domain specific binding; protein binding; enzyme binding; chaperone binding; ubiquitin protein ligase binding; unfolded protein binding; ATPase activity; ribosome binding; calcium ion binding; misfolded protein binding; glycoprotein binding; ATP binding Biological Process: platelet activation; ER-associated protein catabolic process; cerebellum structural organization; unfolded protein response; cerebellar Purkinje cell layer development; cellular response to glucose starvation; platelet degranulation; substantia nigra development; cellular protein metabolic process; unfolded protein response, activation of signaling protein activity; positive regulation of protein ubiquitination; blood coagulation; negative regulation of transforming growth factor beta receptor signaling pathway; ER overload response; positive regulation of cell migration; negative regulation of apoptosis |
| NCBI Summary: | The protein encoded by this gene is a member of the heat shock protein 70 (HSP70) family. It is localized in the lumen of the endoplasmic reticulum (ER), and is involved in the folding and assembly of proteins in the ER. As this protein interacts with many ER proteins, it may play a key role in monitoring protein transport through the cell.[provided by RefSeq, Sep 2010] |
| UniProt Code: | P11021 |
| NCBI GenInfo Identifier: | 14916999 |
| NCBI Gene ID: | 3309 |
| NCBI Accession: | P11021.2 |
| UniProt Secondary Accession: | P11021,Q2EF78, Q9NPF1, Q9UK02, B0QZ61, |
| UniProt Related Accession: | P11021 |
| Molecular Weight: | |
| NCBI Full Name: | 78 kDa glucose-regulated protein |
| NCBI Synonym Full Names: | heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) |
| NCBI Official Symbol: | HSPA5�� |
| NCBI Official Synonym Symbols: | BIP; MIF2; GRP78; HEL-S-89n�� |
| NCBI Protein Information: | 78 kDa glucose-regulated protein; immunoglobulin heavy chain-binding protein; epididymis secretory sperm binding protein Li 89n; endoplasmic reticulum lumenal Ca(2+)-binding protein grp78 |
| UniProt Protein Name: | 78 kDa glucose-regulated protein |
| UniProt Synonym Protein Names: | Endoplasmic reticulum lumenal Ca(2+)-binding protein grp78; Heat shock 70 kDa protein 5; Immunoglobulin heavy chain-binding protein; BiP |
| UniProt Gene Name: | HSPA5�� |
| UniProt Entry Name: | GRP78_HUMAN |