Description
| Product Name: | Human ERO1L Recombinant Protein |
| Product Code: | RPPB3463 |
| Size: | 20µg |
| Species: | Human |
| Target: | ERO1L |
| Synonyms: | ERO1-Like (S. Cerevisiae), Endoplasmic Oxidoreductin-1-Like Protein, Oxidoreductin-1-L-Alpha, ERO1-L-Alpha, ERO1LA, ERO1-L, ERO1 (S. Cerevisiae)-Like, ERO1-Like Protein Alpha, ERO1-Alpha, EC 1.8.4.-, EC 1.8.4, ERO1A, ERO1L. |
| Source: | Escherichia Coli |
| Physical Appearance: | Sterile Filtered colorless solution. |
| Formulation: | The ERO1L solution (1mg/ml) contains 20mM Tris-HCl buffer (pH 8.0) and 10% glycerol. |
| Stability: | Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of time. For long term storage it is recommended to add a carrier protein (0.1% HSA or BSA).Avoid multiple freeze-thaw cycles. |
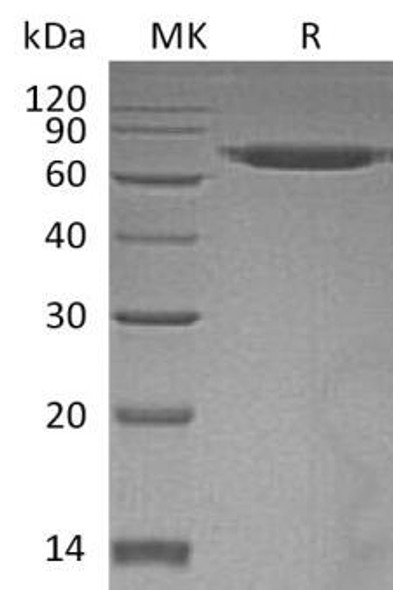
| Purity: | Greater than 90.0% as determined by SDS-PAGE. |
| Amino Acid Sequence: | MGSSHHHHHH SSGLVPRGSH MGSEEQPPET AAQRCFCQVS GYLDDCTCDV ETIDRFNNYR LFPRLQKLLE SDYFRYYKVN LKRPCPFWND ISQCGRRDCA VKPCQSDEVP DGIKSASYKY SEEANNLIEE CEQAERLGAV DESLSEETQK AVLQWTKHDD SSDNFCEADD IQSPEAEYVD LLLNPERYTG YKGPDAWKIW NVIYEENCFK PQTIKRPLNP LASGQGTSEE NTFYSWLEGL CVEKRAFYRL ISGLHASINV HLSARYLLQE TWLEKKWGHN ITEFQQRFDG ILTEGEGPRR LKNLYFLYLI ELRALSKVLP FFERPDFQLF TGNKIQDEEN KMLLLEILHE IKSFPLHFDE NSFFAGDKKE AHKLKEDFRL HFRNISRIMD CVGCFKCRLW GKLQTQGLGT ALKILFSEKL IANMPESGPS YEFHLTRQEI VSLFNAFGRI STSVKELENF RNLLQNIH |
ERO1-like protein alpha (ERO1L) is an essential oxidoreductase, which oxidizes proteins and is necessary for the folding of immunoglobulins. ERO1L covalently binds with PDI (protein disulfide-isomerase) and jointly they generate disulfide bonds between proteins in the endoplasmic reticulum. ERO1L is stimulated by hypoxia, proposing that ERO1L is regulated through the HIF (hypoxia inducible transcrip-tion factor) pathway. At low levels ERO1L is ubiquitously expressed, however at high levels it is expressed in the upper digestive tract and esophagus. In addition, ERO1L is involved in the release of the unfolded cholera toxin from reduced P4HB/PDI in case of infection by V.cholerae, thus having a role in retrotranslocation of the toxin. Furthermore, ERO1L plays an important role in ER stress-induced, CHOP-dependent apoptosis by activating the inositol 1,4,5-trisphosphate receptor IP3R1.
ERO1L Human Recombinant produced in E.coli is a single, non-glycosylated polypeptide chain containing 468 amino acids (24-468) and having a molecular mass of 54.4 kDa.ERO1L is fused to a 23 amino acid His-tag at N-terminus & purified by proprietary chromatographic techniques.
| UniProt Protein Function: | ERO1L: Essential oxidoreductase that oxidizes proteins in the endoplasmic reticulum to produce disulfide bonds. Acts by oxidizing directly P4HB/PDI isomerase through a direct disulfide exchange. Does not act as a direct oxidant of folding substrate, but relies on P4HB/PDI to transfer oxidizing equivalent. Associates with ERP44 but not with GRP54, demonstrating that it does not oxidize all PDI related proteins and can discriminate between PDI and related proteins. Its reoxidation probably involves electron transfer to molecular oxygen via FAD. Acts independently of glutathione. May be responsible for a significant proportion of reactive oxygen species (ROS) in the cell, thereby being a source of oxidative stress. Required for the folding of immunoglobulin proteins. Responsible for the release of the unfolded cholera toxin from reduced P4HB/PDI in case of infection by V.cholerae, thereby playing a role in retrotranslocation of the toxin. Predominantly monomer. May function both as a monomer and a homodimer. Interacts with PDILT. Stimulated by hypoxia; suggesting that it is regulated via the HIF-pathway. Widely expressed at low level. Expressed at high level in upper digestive tract. Highly expressed in esophagus. Weakly expressed in stomach and duodenum. Enzyme activity is tightly regulated to prevent the accumulation of reactive oxygen species in the endoplasmic reticulum. Reversibly down-regulated by the formation of disulfide bonds between the active site Cys-94 and Cys-131, and between Cys- 99 and Cys-104. Glutathione may be required to regulate its activity in the endoplasmic reticulum. Belongs to the EROs family. |
| UniProt Protein Details: | Protein type:EC 1.8.4.-; Oxidoreductase; Secreted; Secreted, signal peptide Chromosomal Location of Human Ortholog: 14q22.1 Cellular Component: endoplasmic reticulum; endoplasmic reticulum lumen; endoplasmic reticulum membrane; intracellular membrane-bound organelle; membrane Molecular Function:disulfide oxidoreductase activity; oxidoreductase activity; protein binding; protein disulfide isomerase activity; protein disulfide oxidoreductase activity Biological Process: chaperone cofactor-dependent protein folding; protein folding; protein modification process; release of sequestered calcium ion into cytosol; response to reactive oxygen species; response to temperature stimulus |
| UniProt Code: | Q96HE7 |
| NCBI GenInfo Identifier: | 50400608 |
| NCBI Gene ID: | 30001 |
| NCBI Accession: | Q96HE7.2 |
| UniProt Secondary Accession: | Q96HE7,Q7LD45, Q9P1Q9, Q9UKV6, A8K9X4, A8MYW1, |
| UniProt Related Accession: | Q96HE7 |
| Molecular Weight: | 54kDa |
| NCBI Full Name: | ERO1-like protein alpha |
| NCBI Synonym Full Names: | endoplasmic reticulum oxidoreductase 1 alpha |
| NCBI Official Symbol: | ERO1A�� |
| NCBI Official Synonym Symbols: | ERO1L; ERO1-L; ERO1LA; Ero1alpha; ERO1-alpha; ERO1-L-alpha�� |
| NCBI Protein Information: | ERO1-like protein alpha |
| UniProt Protein Name: | ERO1-like protein alpha |
| UniProt Synonym Protein Names: | Endoplasmic oxidoreductin-1-like protein; Endoplasmic reticulum oxidoreductase alpha |
| Protein Family: | ERO1-like protein |
| UniProt Gene Name: | ERO1A�� |