Human Autophagy ELISA Kits
Human AMPK (Phosphorylated Adenosine Monophosphate Activated Protein Kinase) CLIA Kit (HUES00231)
- SKU:
- HUES00231
- Product Type:
- ELISA Kit
- ELISA Type:
- CLIA Kit
- Size:
- 96 Assays
- Sensitivity:
- 37.5pg/mL
- Range:
- 62.5-4000pg/mL
- ELISA Type:
- Sandwich
- Synonyms:
- AMPK
- Reactivity:
- Human
- Sample Type:
- Serum, plasma and other biological fluids
- Research Area:
- Autophagy
Description
| Assay type: | Sandwich |
| Format: | 96T |
| Assay time: | 4.5h |
| Reactivity: | Human |
| Detection method: | Chemiluminescence |
| Detection range: | 62.50-4000 pg/mL |
| Sensitivity: | 37.50 pg/mL |
| Sample volume: | 100µL |
| Sample type: | Serum, plasma and other biological fluids |
| Repeatability: | CV < 15% |
| Specificity: | This kit recognizes Human AMPK in samples. No significant cross-reactivity or interference between Human AMPK and analogues was observed. |
This kit uses Sandwich-CLIA as the method. The micro CLIA plate provided in this kit has been pre-coated with an antibody specific to Human AMPK. Standards or samples are added to the appropriate micro CLIA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for Human AMPK and Avidin-Horseradish Peroxidase (HRP) conjugate are added to each micro plate well successively and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human AMPK, biotinylated detection antibody and Avidin-HRP conjugate will appear fluorescence. The Relative light unit (RLU) value is measured spectrophotometrically by the Chemiluminescence immunoassay analyzer. The RLU value is positively associated with the concentration of Human AMPK. The concentration of Human AMPK in the samples can be calculated by comparing the RLU of the samples to the standard curve.
| UniProt Protein Function: | Catalytic subunit of AMP-activated protein kinase (AMPK), an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism. In response to reduction of intracellular ATP levels, AMPK activates energy-producing pathways and inhibits energy-consuming processes: inhibits protein, carbohydrate and lipid biosynthesis, as well as cell growth and proliferation. AMPK acts via direct phosphorylation of metabolic enzymes, and by longer-term effects via phosphorylation of transcription regulators. Also acts as a regulator of cellular polarity by remodeling the actin cytoskeleton; probably by indirectly activating myosin. Regulates lipid synthesis by phosphorylating and inactivating lipid metabolic enzymes such as ACACA, ACACB, GYS1, HMGCR and LIPE; regulates fatty acid and cholesterol synthesis by phosphorylating acetyl-CoA carboxylase (ACACA and ACACB) and hormone-sensitive lipase (LIPE) enzymes, respectively. Regulates insulin-signaling and glycolysis by phosphorylating IRS1, PFKFB2 and PFKFB3. AMPK stimulates glucose uptake in muscle by increasing the translocation of the glucose transporter SLC2A4/GLUT4 to the plasma membrane, possibly by mediating phosphorylation of TBC1D4/AS160. Regulates transcription and chromatin structure by phosphorylating transcription regulators involved in energy metabolism such as CRTC2/TORC2, FOXO3, histone H2B, HDAC5, MEF2C, MLXIPL/ChREBP, EP300, HNF4A, p53/TP53, SREBF1, SREBF2 and PPARGC1A. Acts as a key regulator of glucose homeostasis in liver by phosphorylating CRTC2/TORC2, leading to CRTC2/TORC2 sequestration in the cytoplasm. In response to stress, phosphorylates 'Ser-36' of histone H2B (H2BS36ph), leading to promote transcription. Acts as a key regulator of cell growth and proliferation by phosphorylating TSC2, RPTOR and ATG1/ULK1: in response to nutrient limitation, negatively regulates the mTORC1 complex by phosphorylating RPTOR component of the mTORC1 complex and by phosphorylating and activating TSC2. In response to nutrient limitation, promotes autophagy by phosphorylating and activating ATG1/ULK1. AMPK also acts as a regulator of circadian rhythm by mediating phosphorylation of CRY1, leading to destabilize it. May regulate the Wnt signaling pathway by phosphorylating CTNNB1, leading to stabilize it. Also has tau-protein kinase activity: in response to amyloid beta A4 protein (APP) exposure, activated by CAMKK2, leading to phosphorylation of MAPT/TAU; however the relevance of such data remains unclear in vivo. Also phosphorylates CFTR, EEF2K, KLC1, NOS3 and SLC12A1. |
| NCBI Summary: | The protein encoded by this gene belongs to the ser/thr protein kinase family. It is the catalytic subunit of the 5'-prime-AMP-activated protein kinase (AMPK). AMPK is a cellular energy sensor conserved in all eukaryotic cells. The kinase activity of AMPK is activated by the stimuli that increase the cellular AMP/ATP ratio. AMPK regulates the activities of a number of key metabolic enzymes through phosphorylation. It protects cells from stresses that cause ATP depletion by switching off ATP-consuming biosynthetic pathways. Alternatively spliced transcript variants encoding distinct isoforms have been observed. [provided by RefSeq, Jul 2008] |
| UniProt Code: | Q13131 |
| NCBI GenInfo Identifier: | 254763436 |
| NCBI Gene ID: | 5562 |
| NCBI Accession: | Q13131. 4 |
| UniProt Secondary Accession: | Q13131,O00286, Q5D0E1, Q86VS1, Q9UNQ4, A8MTQ6, B2R7E1 |
| UniProt Related Accession: | Q13131 |
| Molecular Weight: | 65,523 Da |
| NCBI Full Name: | 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
| NCBI Synonym Full Names: | protein kinase AMP-activated catalytic subunit alpha 1 |
| NCBI Official Symbol: | PRKAA1 |
| NCBI Official Synonym Symbols: | AMPK; AMPKa1 |
| NCBI Protein Information: | 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
| UniProt Protein Name: | 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
| UniProt Synonym Protein Names: | Acetyl-CoA carboxylase kinase (EC:2. 7. 11. 27 |
| UniProt Gene Name: | PRKAA1 |
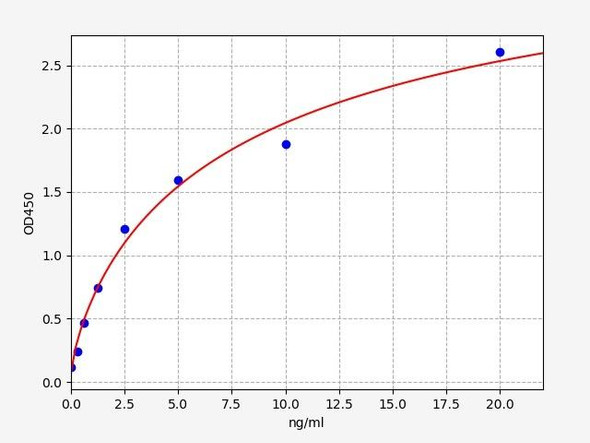
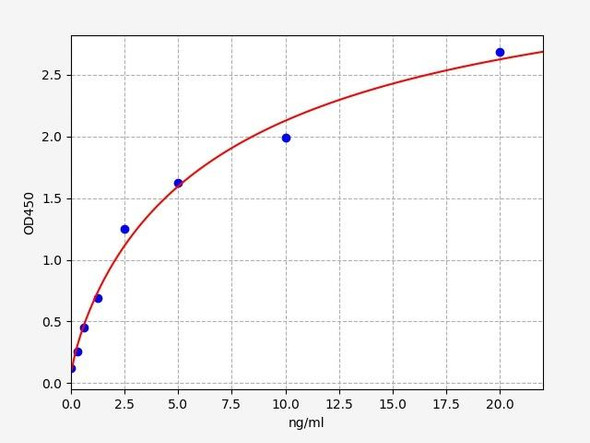
As the RLU values of the standard curve may vary according to the conditions of the actual assay performance (e. g. operator, pipetting technique, washing technique or temperature effects), the operator should establish a standard curve for each test. Typical standard curve and data is provided below for reference only.
| Concentration (pg/mL) | RLU | Average | Corrected |
| 4000 | 58661 59501 | 59081 | 59046 |
| 2000 | 26664 29054 | 27859 | 27824 |
| 1000 | 14528 12440 | 13484 | 13449 |
| 500 | 6231 6979 | 6605 | 6570 |
| 250 | 3322 3164 | 3243 | 3208 |
| 125 | 1635 1527 | 1581 | 1546 |
| 62.50 | 734 776 | 755 | 720 |
| 0 | 34 36 | 35 | -- |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Human AMPK were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Human AMPK were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 222.17 | 342.01 | 1651.96 | 238.78 | 369.06 | 1714.28 |
| Standard deviation | 18.88 | 27.09 | 179.07 | 21.37 | 42.04 | 196.80 |
| C V (%) | 8.50 | 7.92 | 10.84 | 8.95 | 11.39 | 11.48 |
Recovery
The recovery of Human AMPK spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
| Serum (n=5) | 97-113 | 103 |
| EDTA plasma (n=5) | 90-104 | 98 |
| Cell culture media (n=5) | 89-99 | 94 |
Linearity
Samples were spiked with high concentrations of Human AMPK and diluted with Reference Standard & Sample Diluent to produce samples with values within the range of the assay.
| Serum (n=5) | EDTA plasma (n=5) | Cell culture media (n=5) | ||
| 1:2 | Range (%) | 86-101 | 85-100 | 89-106 |
| Average (%) | 93 | 92 | 97 | |
| 1:4 | Range (%) | 87-102 | 94-111 | 87-98 |
| Average (%) | 93 | 101 | 93 | |
| 1:8 | Range (%) | 90-101 | 90-103 | 98-112 |
| Average (%) | 95 | 97 | 106 | |
| 1:16 | Range (%) | 90-105 | 97-111 | 100-116 |
| Average (%) | 97 | 102 | 106 |
An unopened kit can be stored at 4°C for 1 month. If the kit is not used within 1 month, store the items separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
| Micro CLIA Plate(Dismountable) | 8 wells ×12 strips | -20°C, 6 months |
| Reference Standard | 2 vials | |
| Concentrated Biotinylated Detection Ab (100×) | 1 vial, 120 µL | |
| Concentrated HRP Conjugate (100×) | 1 vial, 120 µL | -20°C(shading light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 4°C, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent A | 1 vial, 5 mL | 4°C (shading light) |
| Substrate Reagent B | 1 vial, 5 mL | 4°C (shading light) |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
- Set standard, test sample and control (zero) wells on the pre-coated plate and record theirpositions. It is recommended to measure each standard and sample in duplicate. Note: addall solutions to the bottom of the plate wells while avoiding contact with the well walls. Ensuresolutions do not foam when adding to the wells.
- Aliquot 100µl of standard solutions into the standard wells.
- Add 100µl of Sample / Standard dilution buffer into the control (zero) well.
- Add 100µl of properly diluted sample (serum, plasma, tissue homogenates and otherbiological fluids. ) into test sample wells.
- Cover the plate with the sealer provided in the kit and incubate for 90 min at 37°C.
- Aspirate the liquid from each well, do not wash. Immediately add 100µL of BiotinylatedDetection Ab working solution to each well. Cover the plate with a plate seal and gently mix. Incubate for 1 hour at 37°C.
- Aspirate or decant the solution from the plate and add 350µL of wash buffer to each welland incubate for 1-2 minutes at room temperature. Aspirate the solution from each well andclap the plate on absorbent filter paper to dry. Repeat this process 3 times. Note: a microplatewasher can be used in this step and other wash steps.
- Add 100µL of HRP Conjugate working solution to each well. Cover with a plate seal andincubate for 30 min at 37°C.
- Aspirate or decant the solution from each well. Repeat the wash process for five times asconducted in step 7.
- Add 100µL of Substrate mixture solution to each well. Cover with a new plate seal andincubate for no more than 5 min at 37°C. Protect the plate from light.
- Determine the RLU value of each well immediately.