Metabolism Assays
Glyoxalase I Activity Assay Kit (Colorimetric) (BA0026)
- SKU:
- BA0026
- Product Type:
- Assay
- Detection Method:
- Colorimetric
- Instrument:
- Microplate Reader
- Sample Type:
- Enzyme Preparations Or Biological Samples
- Research Area:
- Metabolism
Description
Glyoxalase I Activity Assay Kit - Information
Assay Genie's Glyoxalase I assay kit provides a sensitive and convenient method for GLO-1 activity determination. The method involves monitoring the increase in the product of the GLO-1 reaction, S-lactoylglutathione, by measuring the change in absorbance at 240 nm.Applications
For quantitative determination of glyoxalase activity. Note: use a UV plateGlyoxalase I Activity Assay Kit - Key Features
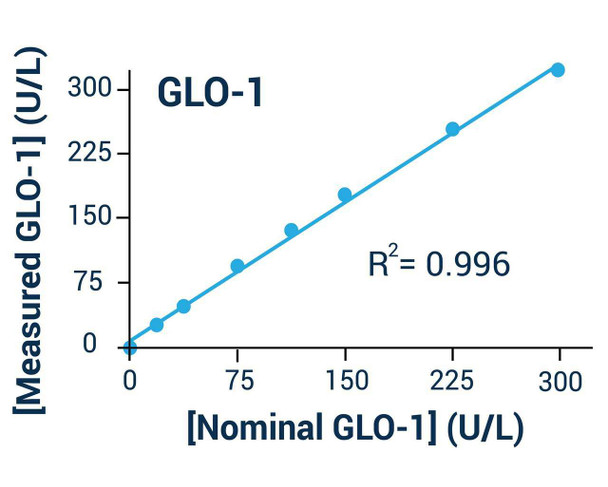
- Fast and sensitive. Linear detection range (20 uL sample): 0.4 to 80 U/L for 30 min reaction. Detection Limit of 0.1 U/L for 120 min reaction.
- Convenient and high-throughput. Homogeneous "mix-incubate-measure" type assay. Can be readily automated on HTS liquid handling systems for processing thousands of samples per day.
Glyoxalase I Activity Assay Kit - Data Sheet | |
| Kit Includes | Assay Buffer (pH 6.6): 20 mL Substrate: 1 mL 96 well UV Titer Plate 1 Plate Cosubstrate: 1 mL |
| Kit Requires | Perchloric Acid, pipetting devices and accessories and plate reader. |
| Method of Detection | OD240nm |
| Detection Limit | 4 U/L |
| Samples | Enzyme preparations or biological samples |
| Species | All |
| Protocol Length | 30 min |
| Size | 100 tests |
| Storage | Store the plate at room temperature and other components at - 20°C |
| Shelf Life | 12 months |
More Details
GLYOXALASE I (GLO-1), a lactoylglutathione lyase also known as methylglyoxalase, aldoketomutase, ketone-aldehyde mutase, and (R)-S-lactoylglutathione methylglyoxal-lyase, is an enzyme that catalyzes the isomerization of hemithioacetal adducts which are formed in spontaneous reactions between glutathionyl groups and aldehydes. The primary physiological function of glyoxalase I is the detoxification of methylglyoxal, a reactive 2-oxoaldehyde that is cytostatic at low concentrations and cytotoxic at millimolar concentrations. Glyoxalase I is a target for the development of pharmaceuticals against bacteria, protozoans and human cancer. Simple, direct and automation-ready procedures for measuring GLO-1 activity in biological samples are highly desirable in research and drug discovery.Aliases for GLO1 Gene
- Glyoxalase I
- S-D-Lactoylglutathione Methylglyoxal Lyase
- Glyoxalase Domain Containing 1
- Ketone-Aldehyde Mutase
- Methylglyoxalase
- Aldoketomutase
- EC 4.4.1.5
- Glx I
Entrez Gene Summary for GLO1 Gene
The enzyme encoded by this gene is responsible for the catalysis and formation of S-lactoyl-glutathione from methylglyoxal condensation and reduced glutatione. Glyoxalase I is linked to HLA and is localized to 6p21.3-p21.1, between HLA and the centromere. [provided by RefSeq, Jul 2008UniProtKB/Swiss-Prot for GLO1 Gene LGUL_HUMAN,Q04760Catalyzes the conversion of hemimercaptal, formed from methylglyoxal and glutathione, to S-lactoylglutathioneInvolved in the regulation of TNF-induced transcriptional activity of NF-kappa-B. Required for normal osteoclastogenesis.
Protein details for GLO1 Gene (UniProtKB/Swiss-Prot)
- Protein Symbol:Q04760-LGUL_HUMAN
- Recommended name:Lactoylglutathione lyase
- Protein Accession:Q04760
Protein attributes for GLO1 Gene
- Size:184 amino acids
- Molecular mass:20778 Da
- Cofactor:Name=Zn(2+); Xref=ChEBI:CHEBI:29105;
- Quaternary structure:Homodimer.
- SequenceCaution:Sequence=BAD93038.1; Type=Erroneous initiation; Note=Translation N-terminally shortened.; Evidence={ECO:0000305};
Post-translational modifications for GLO1 Gene
- Exists in a nitric oxide (NO)-modified form. The exact nature of the modification is unknown, but it suppresses the TNF-induced transcriptional activity of NF-kappa-B.Q04760-LGUL_HUMAN
- Glutathionylation at Cys-139 inhibits enzyme activity.Q04760-LGUL_HUMAN
- Phosphorylated at Thr-107 in the presence of CaMK2. However, this is a consensus site for phosphorylation by CK2 so phosphorylation may be mediated by CK2 rather than CaMK2. Phosphorylation is induced by TNF and suppresses the TNF-induced transcriptional activity of NF-kappa-B.Q04760-LGUL_HUMAN
- Ubiquitination at Lys 44, Lys 140, and Lys 148 NX_Q04760
- Modification sites at PhosphoSitePlus Q04760