Description
GenieHTS Potassium Ion Channel Assay
High-throughput, no wash thallium (Tl⁺) assay. An optimal solution for measuring flux through potassium (K⁺), sodium (Na⁺), and non-selective cation channels and their effectors including transporters and GPCRs.
| Product Name: | GenieHTS Potassium Ion Channel Assay |
| Product Code: | ASIB003 |
| Product Size: | 10 plates |
| Excitation: | 490 nm |
| Emission: | 515 nm |
| Molecular Weight: | 840 |
| Component Name | Size | Storage |
| GenieHTS Potassium Ion Channel | Lyophilized (10) | -20°C |
| DMSO | 225μL | 4°C |
| Dye Solvent | 4mL | 4°C |
| 10X Assay Buffer | 20mL | 4°C |
| TRS | 4mL | 4°C |
| Probenecid Solution | 4mL | 4°C |
| 10X Chloride-Free Stimulus Buffer | 10mL | 4°C |
| 10X High-Potassium Stimulus Buffer | 10mL | 4°C |
| Thallium Sulfate Solution (50nM) | 20mL | 20-25°C |
Materials needed but not provided
- Compounds to be tested.
- Buffers and solvents for dissolution.
- Reagents necessary for cell culture.
- A fluorescence plate reader ~ 490 nm /~ 520 nM.
- Plate reader capable of collect kinetic data (1 Hz) e.g. WaveFront Panoptic, Hamamatsu FDSS, Molecular Devices FLIPR and Molecular Devices FlexStation.
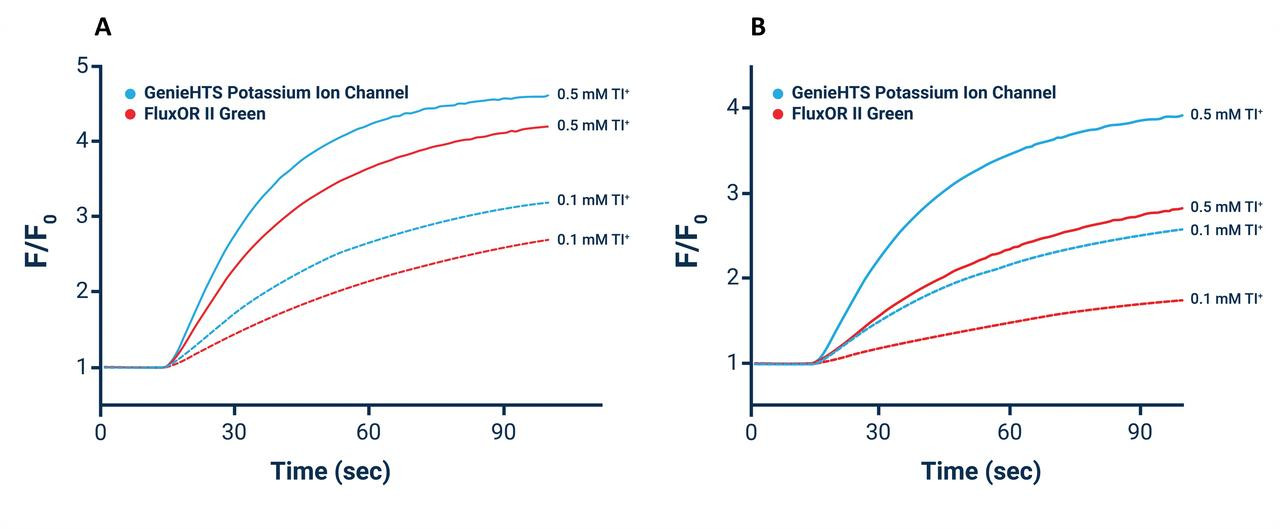
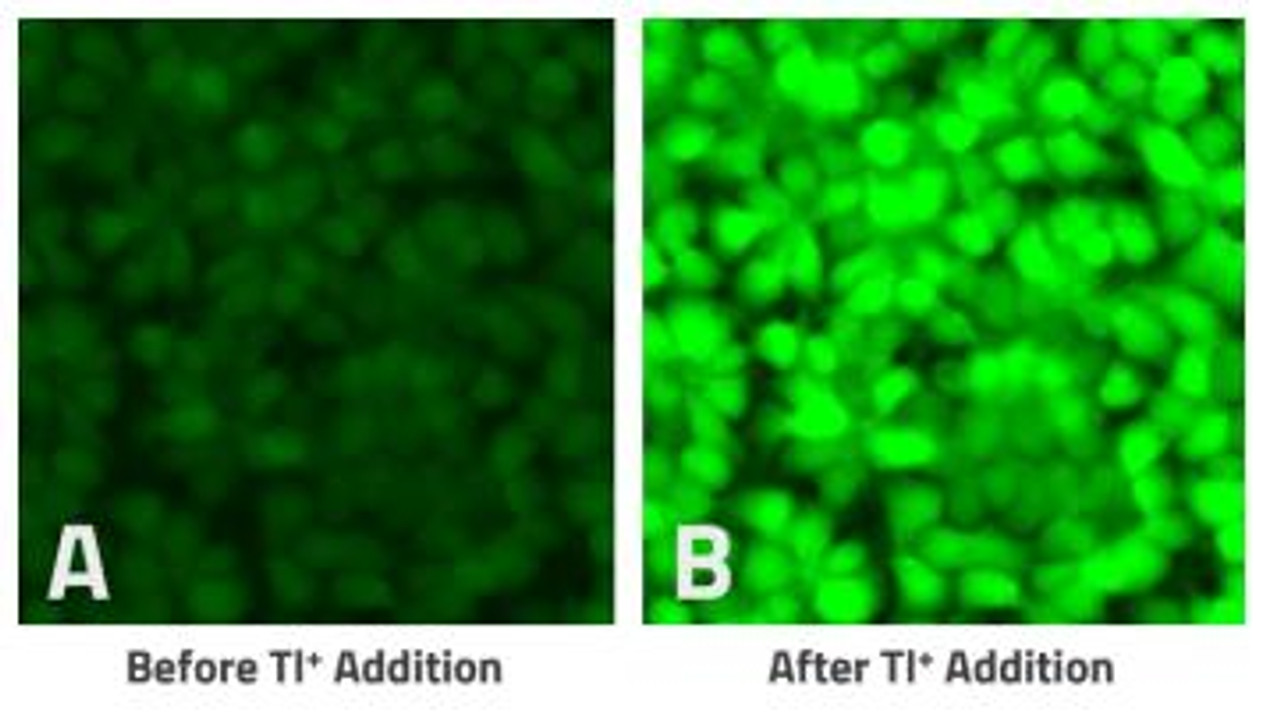
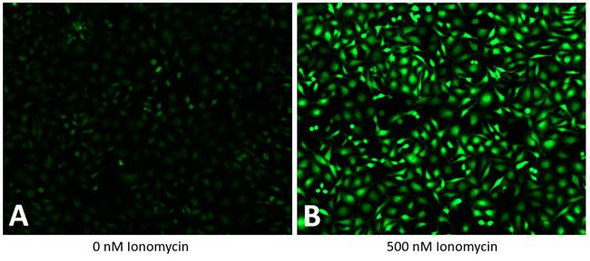
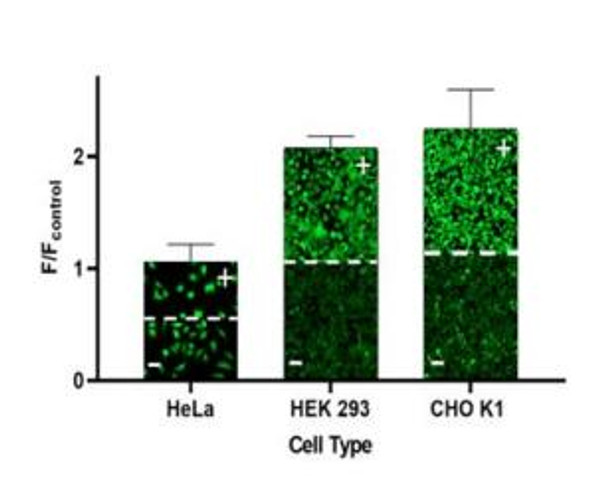
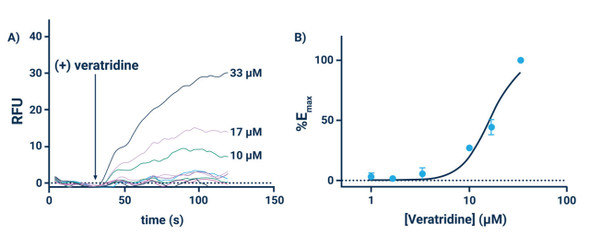
The Assay Genie Potassium Ion Channel Assay is a Thallium total assay solution for multi-well plate-based, high- throughput measurements of Tl+ flux through K+ , Na+ , non-selective cation channels, and some Na+ or K+ transporters. The GenieHTS Potassium Ion Channel Assay is also useful for a wide range of effectors of ion channels and transporters including G protein-coupled receptors, lipid kinases and protein kinases. In multi-well, plate- based formats, the GenieHTS Potassium Ion Channel can be used to discover and characterize the effects of many tens-of-thousands of compounds and environmental factors on effectors of Tl+ flux. Over the past 15 years, fluorescence-based measures of Tl+ flux have brought about the discovery of small-molecule modulators of a host of Ion channels, transporters, GPCRS and other targets of interest for both drug discovery and basic research. GenieHTS Potassium Ion Channel provides all the reagents necessary for use as a washed or no-wash assay with adherent or non-adherent cells. The optional use of a probenecid solution and an extracellular background masking solution offers the ultimate in compatibility for cells types which are difficult to load with fluorescent Tl+ indicators (e.g. Chinese Hamster Ovary, CHO cells) and when performing assays in complete, serum-containing cell culture medium is desired.
Wash Method Adherent Cells
The instructions given below are for one, 384-well microplate. Certain aspects of the instructions may need to be altered, as appropriate, for multiple microplates or other assay formats (e.g. 96-well microplates or non-adherent cells). The Potassium Ion Channel indicator and Potassium Ion Channel indicator-containing solutions should be protected from direct light.
- Add 20 μL DMSO to the tube containing Potassium Ion Channel Indicator.
- Vortex until Potassium Ion Reagent is fully dissolved.
- Add appropriate volume of water to a 15 mL centrifuge tube.
- Add 1 mL of 10X Assay Buffer to tube from step 3.
- Add 200 μL of Dye Solvent to the tube from step 4.
- If desired add 200 μL of Probenecid Solution to the tube from step 5.
- Add 20 μL of Potassium Ion Channel Solution from step 2 to the tube from step 6.
- Briefly vortex the tube from step 7 to mix.
- Remove the cell-culture medium from the 384-well microplate containing the cells of interest.
- Add 20 μL per well of the Dye Loading Solution from step 8 to the microplate from step 9.
- Incubate the microplate containing the cells and Dye Loading Solution for 1 hour at room temperature.
- Prepare Wash Solution in a 15 mL centrifuge tube by adding the appropriate amounts of water, 10X Assay .Buffer and other components if desired as shown in Table 3.
- Briefly vortex the tube from step 12 to mix.
- Remove Dye Loading Solution from microplate in step 11.
- Add 20 μL per well of the Wash Solution prepared in step 13 to the microplate from step 14.
- Prepare Potassium Ion Stimulus Solution in a 15 mL centrifuge tube by adding the appropriate amounts of water, 10X Stimulus Buffer and Thallium Sulfate Solution as shown in Table 4*.
- Briefly vortex the tube from step 16 to mix.
- Add 20 μL per well of the Potassium Ion Stimulus Solution from step 17 to an empty 384-well microplate.
- Transfer the washed, dye-loaded, cell-containing microplate from step 15 and the Potassium Ion Stimulus Solution micro- plate from step 17 to a kinetic-imaging plate reader (e.g. WaveFront Panoptic, Hamamatsu FDSS, Molecular Devices FLIPR or Molecular Devices FlexStation).
- Acquire data using an excitation wavelength of ~490 nm, an emission wavelength of ~520 nm and an acquisition frequency of 1 Hz. Begin data acquisition and after 10 seconds add 5 μL of the Potassium Ion Stimulus Solution to the cell containing plate and continue data acquisition for an additional 90 seconds**.
**The timing of and volume of Potassium Stimulus Solution addition may vary. In some cases, experiments may include the addition of other solutions to the cell-containing microplate prior to the addition of the Potassium Stimulus Solution. In these cases, the volume of the Potassium Stimulus Solution addition should be altered to account for the additional volume of solution in the cell-containing microplate.
Dye Loading Solution (Wash Method)
| Component | Method 1 | Method 2 |
| GenieHTS Potassium Ion Channel Solution | 20μL | 20μL |
| Dye Solvent | 200μL | 200μL |
| 10X Assay Buffer | 1mL | 1mL |
| Probenecid Solution* | - | 200μL |
| Water | 8.8mL | 8.6mL |
| Total | 10mL | 10mL |
* Probenecid may be included in the Dye Loading Solution to aid dye retention. This may be particularly important in certain cell lines (e.g. CHO cells). However, caution is advised when using Probenecid as it may have undesirable effects on assay performance for the target of interest.
Wash Solution (Wash Method)
| Component | Method 1 | Method 2 | Method 3 | Method 4 |
| 10X Assay Buffer | 1mL | 1mL | 1mL | 1mL |
| TRS* | - | 200μL | - | 200μL |
| Probenecid Solution | - | - | 200μL | 200μL |
| Water | 9mL | 8.8mL | 8.8mL | 8.6mL |
| Total | 10mL | 10mL | 10mL | 10mL |
*TRS contains a membrane-impermeant dye useful for masking extracellular fluorescence. Caution is advised when using TRS or other extracellular masking solutions as they may have undesirable effects on assay performance for the target of interest.
Potassium Ion Stimulus Solution (Wash Method)
| Component | Method 1 | Method 2 |
| 10X Chloride-Free Stimulus Buffer | 1mL | 0.5 mL |
| 10X High-Potassium Stimulus Buffer | - | 0.5 mL |
| Thallium Sulfate Solution (50 mM) | 0.5 mL | 0.5 mL |
| Water | 8.5 mL | 8.5 mL |
| Total | 10 mL | 10 mL |
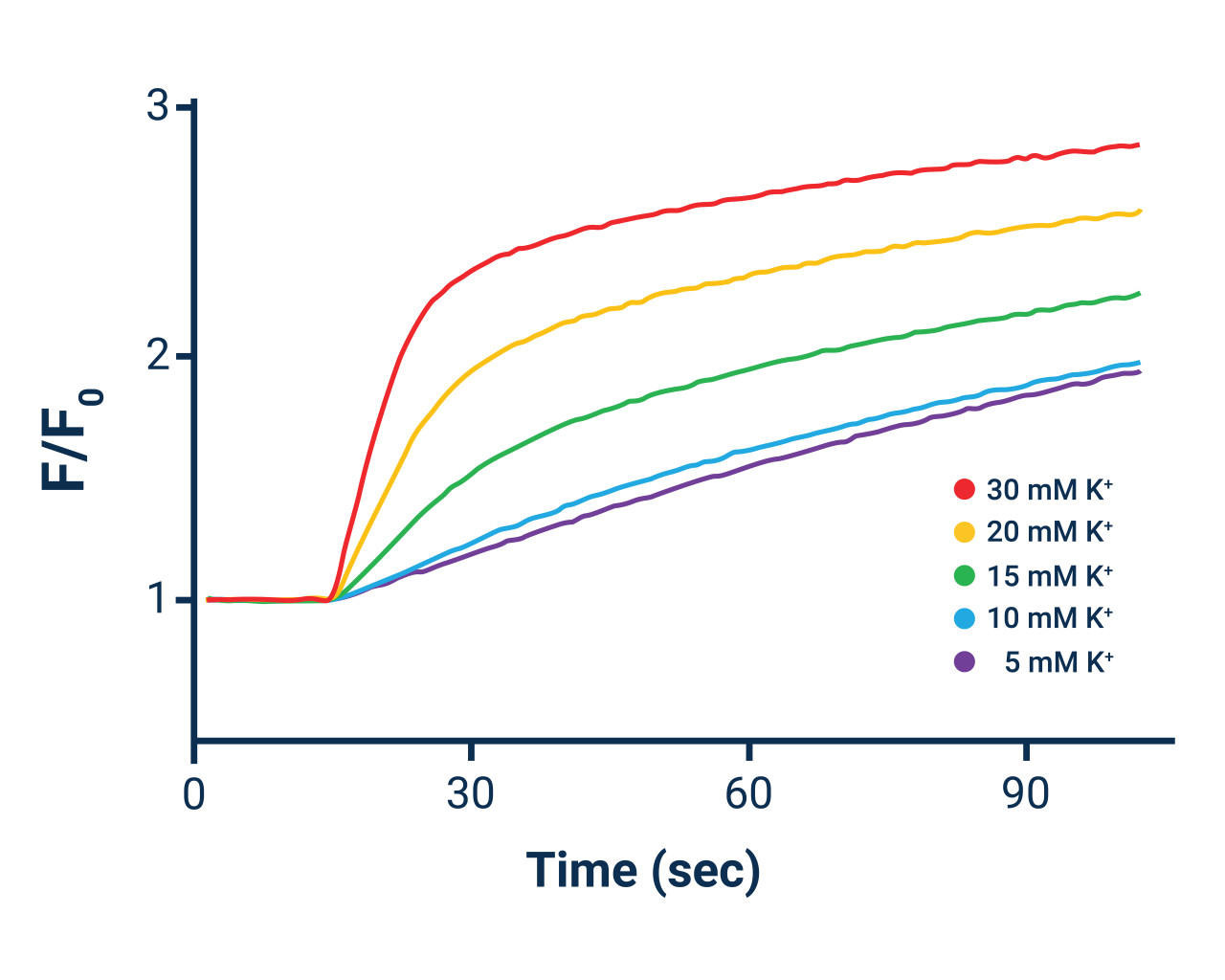
*The above table provides two examples of Potassium Ion Stimulus solutions useful for many types of non-voltage-gated and voltage- gated monovalent cation channels and transporters. Elevation of extracellular potassium (Method 2) may provide superior results for some voltage-gated channels. The concentration of Potassium Ion in the stimulus solution may be varied to achieve the desired result. The final Potassium Ion concentration in the cell-containing microplate post-potassium Ion stimulus buffer addition should not exceed 4.8 mM due to the ~ 5 mM solubility limit of thallium in chloride-containing solutions.
No-wash Method — Adherent Cells
- Add 20 μL DMSO to the tube containing Potassium Ion Channel indicator.
- Vortex until the Vortex until Potassium Ion Reagent is fully dissolved.
- Add appropriate volume of water (Table 4) to a 15 mL centrifuge tube.
- Add 1 mL of 10X Assay Buffer to tube from step 3.
- Add 400 μL of Dye Solvent to the tube from step 4.
- Add 400 μL of TRS to the tube from step 5.
- If desired add 400 μL of Probenecid Solution to the tube from step 6.
- Add 20 μL of GenieHTS Potassium Ion Indicator Solution from step 2 to the tube from step 7.
- Briefly vortex the tube from step 8 to mix.
- Add 20 μL per well of the Dye Loading Solution from step 9 to the cell-containing microplate. Do not remove the cell culture medium.
- Incubate the microplate containing the cells and Dye Loading Solution for 1 hour at 37°C in a cell culture incubator.
- Prepare Potassium Stimulus Solution in a 15 mL centrifuge tube by adding the appropriate amounts of water, 10X Stimulus Buffer and Thallium Sulfate Solution as shown in Table 6*.
- Briefly vortex the tube from step 12 to mix.
- Add 20 μL per well of the Potassium Stimulus Solution from step 13 to an empty 384-well microplate.
- Transfer the dye-loaded, cell-containing microplate from step 11 and the Potassium Stimulus Solution microplate from step 14 to a kinetic-imaging plate reader (e.g. WaveFront Panoptic, Hamamatsu FDSS, Molecular Devices FLIPR or Molecular Devices FlexStation).
- Acquire data using an excitation wavelength of ~490 nm, an emission wavelength of ~520 nm and an acquisition frequency of 1 Hz. Begin data acquisition and after 10 seconds add 10 μL of the Potassium Stimulus Solution to the cell- containing plate and continue data acquisition for an additional 90 seconds**.
**The timing of and volume of stimulus solution addition may vary. Some experiments may include the addition of other solutions to the cell-containing microplate prior to the addition of the stimulus solution. In these cases, the volume of the stimulus solution addition should be altered to account for the additional volume of solution in the cell-containing microplate.
Potassium Ion Stimulus Solution (No-wash Method)
| Component | Method 1 | Method 2 |
| 10X Chloride-Free Stimulus Buffer | 1mL | 0.5 mL |
| 10X High-Potassium Stimulus Buffer | - | 0.5 mL |
| Thallium Sulfate Solution (50 mM) | 0.5 mL | 0.5 mL |
| Water | 8.5 mL | 8.5 mL |
| Total | 10 mL | 10 mL |
*The above table provides two examples of Potassium Ion Stimulus solutions useful for many types of non-voltage-gated and voltage- gated monovalent cation channels and transporters. Elevation of extracellular potassium (Method 2) may provide superior results for some voltage-gated channels. The concentration of Potassium Ion in the stimulus solution may be varied to achieve the desired result. The final Potassium Ion concentration in the cell-containing microplate post-potassium Ion stimulus buffer addition should not exceed 4.8 mM due to the ~ 5 mM solubility limit of thallium in chloride-containing solutions.