Therapeutic Drug Monitoring
Evolocumab (Repatha® )Free Drug -Quantitative ELISA Kit
- SKU:
- HUMB00064
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Evolocumab (Repatha®)
- Research Area:
- Obesity
Description
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit (HUMB00064)
The Assay Genie Evolocumab ELISA has been developed for the quantitative determination of Evolocumab in serum and plasma.
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit Test Principle
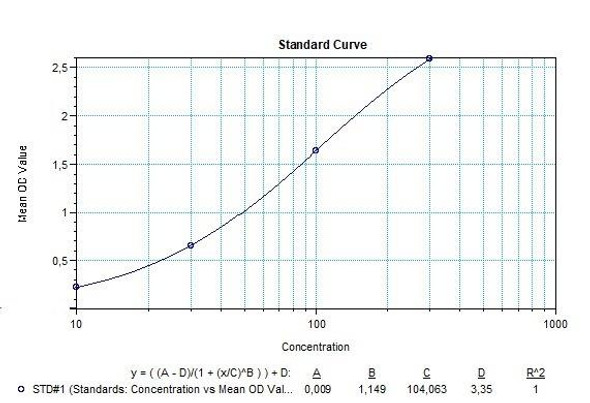
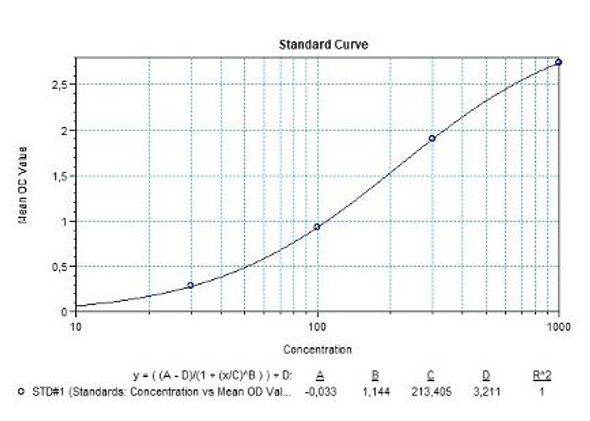
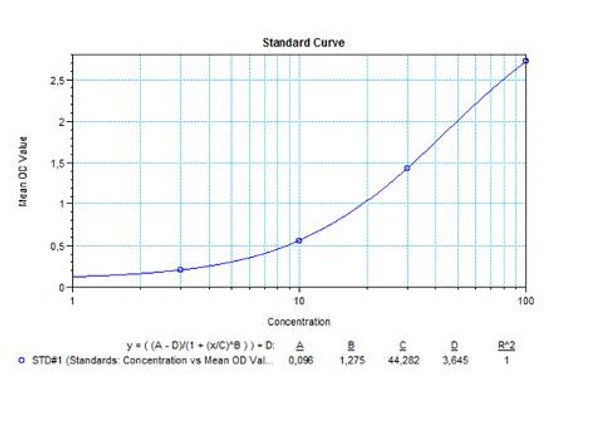
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the reactant for evolocumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to evolocumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of evolocumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit Product Information
| Information | Description |
| Application | Free Drug |
| Required Volume (µl) | 10 |
| Total Time (min) | 140 |
| Sample Typle | Serum, plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | 6.25 |
| Recovery | 85-115% |
| Shelf Life (year) | 1 |
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit - General Information
Proprotein convertase subtilisin/kexin type 9 (PCSK9) was discovered in 2003 and subsequently emerged as a novel target for LDL-C-lowering therapy. By inactivating PCSK9, evolocumab upregulates LDL receptors causing increased catabolism of LDL-C and the consequent reduction of LDL-C levels in blood. Overall, evolocumab has had notable efficacy, with LDL-C reduction ranging from 53% to 75% in monotherapy and combination therapies, and is associated with minor adverse effects.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) increases plasma low-density lipoprotein cholesterol (LDL-C) by decreasing expression of the LDL receptor on hepatic cells. Evolocumab is a human monoclonal immunoglobulin G2 that binds specifically to human PCSK9 to reduce LDL-C. Evolocumab exhibits nonlinear kinetics as a result of binding to PCSK9. Elimination is predominantly through saturable binding to PCSK9 at lower concentrations and a nonsaturable proteolytic pathway at higher concentrations.
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit Contents
| Size | Kit Contents |
| 1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
| 0,3 mL (each) | Standards A-F (10x) Standard A: 1000 ng/mL Standard B: 500 ng/mL Standard C: 250 ng/mL Standard D: 125 ng/mL Standard E: 62.5 ng/mL Standard F: 0 ng/mL Used for the standard curve and control. Contains evolocumab, human serum and stabilizer, <0,1% NaN3. Standards are prepared concentrated (10x). |
| 0,3 mL (each) | Controls Control low and high levels (10x) Contains human serum and stabilizer, <0,1% NaN3. Controls are prepared concentrated (10x). |
| 2x50 mL | Assay buffer Ready to use. Blue coloured. Contains proteins, <0,1% NaN3. |
| 1x12 mL | Horse radish peroxidase conjugated probe. Ready to use. Red coloured. Contains HRP conjugated probe, stabilizer and preservatives. |
| 1x12 mL | TMB substrate solution. Ready to use. Contains 3,3′,5,5′- Tetramethylbenzidine (TMB). |
| 1x12mL | TMB stop solution. Ready to use. 1N HCl |
| 1x50mL | Wash buffer (20x). Prepared concentrated (20x) and should be diluted with the dilution rate given in the “Pretest setup instructions” before the test. Contains buffer with tween 20. |
| 2x1 | Adhesive Foil. For covering microtiter plate during incubation |
Evolocumab (Repatha® ) Free Drug -Quantitative ELISA Kit Protocol
| 1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
| 2 | Pipette 100 µL of each diluted Standards, Low level control, High level control and samples into the respective wells of microtiter plate Wells A1: Standard A B1: Standard B C1: Standard C D1: Standard D E1: Standard E F1: Standard F G1: Low level control H1: High level control A2 and on: Samples |
| 3 | Cover the plate with adhesive foil. Briefly mix contents by gently shaking the plate. Incubate 60 minutes at room temperature (18-25°C) |
| 4 | Remove adhesive foil. Discard incubation solution. Wash plate three times each with 300 µL Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel |
| 5 | Pipette 100 µL Conjugate into each well |
| 6 | Cover the plate with adhesive foil. Incubate 60 minutes at room temperature (18-25°C) |
| 7 | Remove adhesive foil. Discard incubation solution. Wash plate three times each with 300 µL Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel |
| 8 | Pipette 100 µL Substrate into each well |
| 9 | Incubate 20 minutes without adhesive foil at room temperature (18-25°C) in the dark |
| 10 | Stop the substrate reaction by adding 100 µL Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 11 | Measure optical density with a photometer at OD 450nm with reference wavelength 650 nm (450/650 nm) within 30 minutes after pipetting the Stop Solution. |