Description
Description
The Getein COVID-19 Rapid POC (Point-of-Care) kit is a One Step Test for Novel Coronavirus (2019-nCoV) IgM/IgG antibody (Colloidal Gold) detection and is intended for the qualitative detection of 2019-Novel Coronavirus IgM and IgG antibody in serum, plasma, fingertip blood or whole blood samples of pneumonitis patients or suspected cases.
Summary
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by 2019-nCoV, a new strain of coronavirus that has not been previously identified in humans. The disease is primarily spread between people via respiratory droplets from infected individuals when they cough or sneeze. Time from exposure to onset of symptoms is generally between 2 and 14 days. The disease may initially present with few or no symptoms, or may develop into fever, coughing, shortness of breath, pain in the muscles and tiredness. Further development may include pneumonia and acute respiratory distress syndrome.
Detection of 2019-nCoV IgM and IgG antibodies in human blood can be used as an auxiliary means for screening of COVID-19.
Principle
The test uses mixed recombinant 2019-nCoV nucleocapsid protein (N protein) and spike protein (S protein) both conjugated with colloidal gold and anti-human IgM and IgG antibody coated on different test lines respectively. After the samples has been applied to the test strip, the gold-labelled recombinant 2019-nCoV N protein and S protein will bind with 2019-nCoV IgM or IgG antibody in sample and form marked antigen-antibody complexes. These complexes move to the
test card detection zone by capillary action. Then marked antigen-antibody complexes will be captured on different test lines by anti-human IgM and IgG antibody resulting in purplish red streaks on the test lines. The color intensity of each test line increases in proportion to the amount of 2019-nCoV IgM and IgG antibody in sample.
Kit contents
| 25 tests/box |
| Each kit contains
|
| A test card contains A plastic shell and a reagent strip which is composed of a sample pad, a colloidal gold pad (coated with recombinant 2019-nCoV N protein and S protein), nitrocellulose membrane with two test lines (these two lines are coated with anti-human IgM and IgG antibody respectively), the control line (coated with anti recombinant protein tag protein ), absorbent paper and liner. |
| Sample diluent composition Phosphate buffered saline, proteins, detergent, preservative, stabilizer |
Storage & Stability
Store the test card at 4-30℃ with a valid period of 24 months. Use the test card within 1 hour once the foil pouch is opened. Store the sample diluent at 0-30℃ with a valid period of 24 months.
Store the sample diluent at 2-8℃ for better results.
Sample collection
| Sample collection |
| 1. Sample should be human serum, plasma, fingertip blood and whole blood, other body fluid and samples may cause incorrect or inaccurate results. |
| 2. Venous blood should be collected under sterile condition at any time of a day. |
| 3. Suggest using serum or plasma for better results. |
| 4. Heparin, sodium citrate and EDTA can be used as anticoagulant for plasma, fingertip blood and whole blood sample. |
| 5. Serum, plasma, fingertip blood or whole blood sample should be tested within 4 hours after blood collection in room temperature. If testing will be delayed, serum and plasma may be stored up to 5 days at 2-8 ℃ or stored for 6 months at -20 ℃ before testing (fingertip blood and whole blood sample may be stored up to 3 days at 2-8 ℃). Do not heat the samples and discard hemolyzed samples. |
| 6. Bring all samples to room temperature (15-30 ℃) before use. |
| 7. SAMPLE VOLUME: 10 μL of serum and plasma sample, 20 μL of fingertip blood and whole blood sample. |
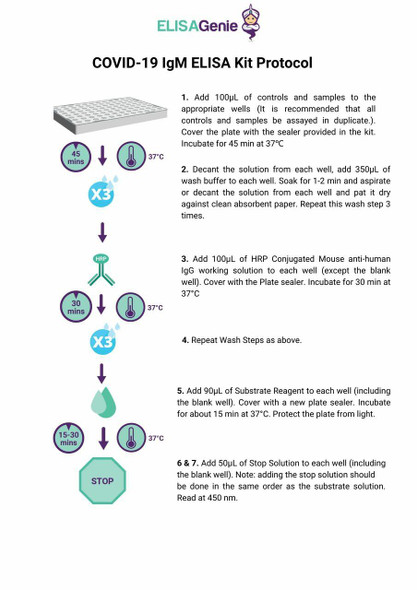
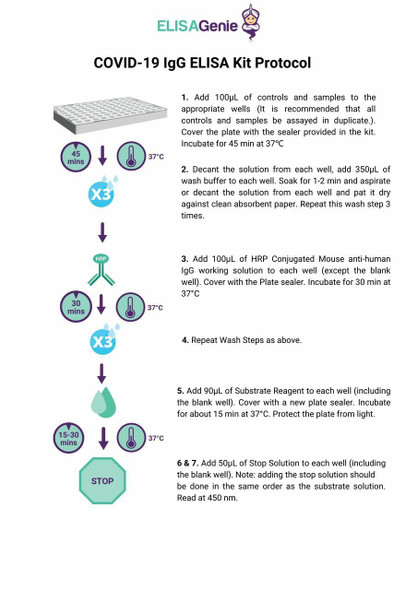
Test procedure
Read the manual carefully before using and operate according to the manual to avoid incorrect results.
| Protocol |
| 1. Collect specimens according to user manual. |
| 2. Test card, sample and reagent should reach to room temperature (15-30 ℃ ) before test. |
| 3. Remove the test card from the sealed pouch immediately before use. Label the test card with patient or control identification. |
| 4. Put the test card on a clean table, horizontally placed. |
| 5. Using sample transfer pipette, deliver sample (10 μL of serum and plasma sample, 20 μL of fingertip blood and whole blood sample) into the sample port on the test card. Then add 2 or 3 drops of sample diluent immediately. |
| 6. Read the results visually in 10-15 min. |
Test results
| Positive Results |
| 3 purplish red bands appear, one at the control area (C) and two at the test lines (T1, T2). The result indicates that the sample contains both 2019-nCoV IgM and IgG antibody. |
| 2 purplish red bands appear, one at the control area (C) and one at the test line (T1). The result indicates that the sample contains 2019-nCoV IgM antibody. |
| 2 purplish red bands appear, one at the control area (C) and one at the test line (T2). The result indicates that the sample contains 2019-nCoV IgG antibody. |
| Negative Results |
| A single purplish red band appears at the control area (C) without any other band at test lines. The result indicates that the sample does not contain 2019-nCoV IgM or IgG antibody |
| Invalid Results |
| If no colored band appears in the control area (C) in 5-10 min, the test result is invalid. The test should be repeated and if the same situation happened again, please stop using this batch of products and contact your supplier.、 |
Limitations
1. The test is for in vitro diagnostic use only.
2. The test results of this kit are for clinical reference only. The clinical diagnosis and treatment of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests, and treatment response.
Disclaimer
While we believe this kit is to be an effective indicator of infection we cannot guarantee 100% accuracy so the patient should still be advised to follow government guidelines for those exhibiting symptoms and those who aren’t regarding hygiene, self-isolation and other measures even if the test is negative.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- This kit is for Professional-Use-Only.
Additional Information
COVID-19 Rapid POC Citations
| Green et al. | Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19 | The Centre for Evidence-Based Medicine (2020) | View Citation |
Citations
| Green et al. | Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19 | The Centre for Evidence-Based Medicine (2020) | View Citation |