Therapeutic Drug Monitoring
Certolizumab (Cimzia®) ELISA Kit
- SKU:
- HUMB00017
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Certolizumab (Cimzia®)
- Research Area:
- Anti-TNF Alpha
Description
Certolizumab (Cimzia®) ELISA Kit
Enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of Certolizumab (CIMZIA®) in human serum and plasma. This Assay Genie kit has been developed for the quantitative analysis of free Certolizumab in serum and plasma samples.
Certolizumab (Cimzia®) ELISA Kit test principle
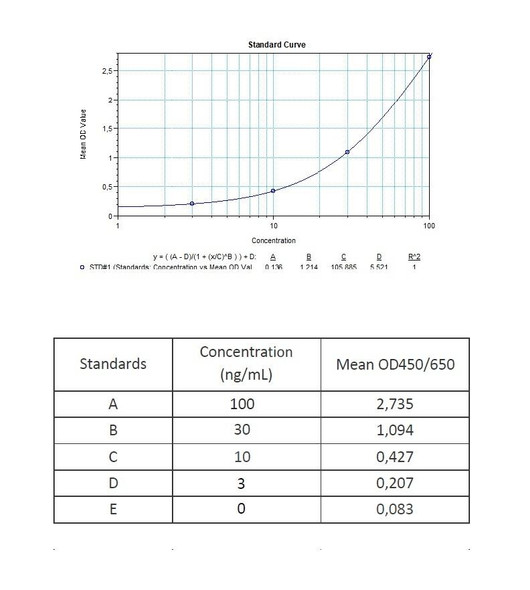
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtitre plate coated with the reactant for certolizumab pegol (Cimzia®). After incubation, the wells are washed. A horse radish peroxidase (HRP) conjugated probe is added and binds to certolizumab pegol captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of certolizumab pegol in the sample or standard. Results of samples can be determined directly using the standard curve.
Certolizumab (Cimzia®) ELISA Product Information
| Description | Information |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 70 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 37 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-Tumour Necrosis Factor Alpha Cimzia |
Certolizumab (Cimzia®) - Key Information
Certolizumab (Cimzia®) mode of action
Certolizumab pegol is a polyethylene glycolylated (PEGylated) Fab’ fragment of a humanized monoclonal antibody, which functions as an anti-TNF (anti-tumour necrosis factor) drug. Certolizumab pegol binds to free and membrane bound human TNF alpha with a high affinity (KD of 90pM) and neutralizes its activity. The extent of neutralization is also dose dependent. Certolizumab pegol also inhibits the release of lipopolysaccharide induced IL-1 beta from monocytes. TNF alpha is a key pro-inflammatory cytokine with a central role in inflammatory processes in which elevated levels have been observed in patients with Rheumatoid Arthritis and Crohn's Disease.
Certolizumab (Cimzia®) uses
Certolizumab (Cimzia®) blocks the action of TNF protein and so reduces inflammation in various conditions including rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. TNF alpha is a key pro-inflammatory cytokine with a central role in inflammatory processes. The biological activity associated with TNF alpha includes the upregulation of cellular adhesion molecules and chemokines, upregulation of major histocompatibility complex (MHC) class I and class II molecules, and direct leukocyte activation. TNF alpha stimulates the production of downstream inflammatory mediators, including interleukin-1, prostaglandins, platelet activating factor, and nitric oxide.
Certolizumab (Cimzia®) treatment
After treatment with certolizumab pegol, patients with Crohn's disease demonstrated a decrease in C-reactive protein (CRP) levels. Certolizumab pegol selectively neutralizes TNF alpha, it does not bind to TNF-alpha. Since, certolizumab is only a Fab' fragment and is therefore missing the Fc region, it does not fix complement or cause antibody-dependent cell-mediated cytotoxicity.
Furthermore, apoptosis of monocytes or lymphocytes, or neutrophil degranulation have not been observed in vitro. There is a linear relationship between dose administered and Cmax and AUC. A mean Cmax of approximately 43 to 49 mcg/mL occurred at Week 5 during the initial loading dose period using the recommended dose regimen for the treatment of patients with rheumatoid arthritis (400 mg sc at Weeks 0, 2 and 4 followed by 200 mg every other week).
Tmax, SubQ dose = 54 - 171 hours; Bioavailability, SubQ dose = 80% (range of 76% - 88%).
Certolizumab (Cimzia®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
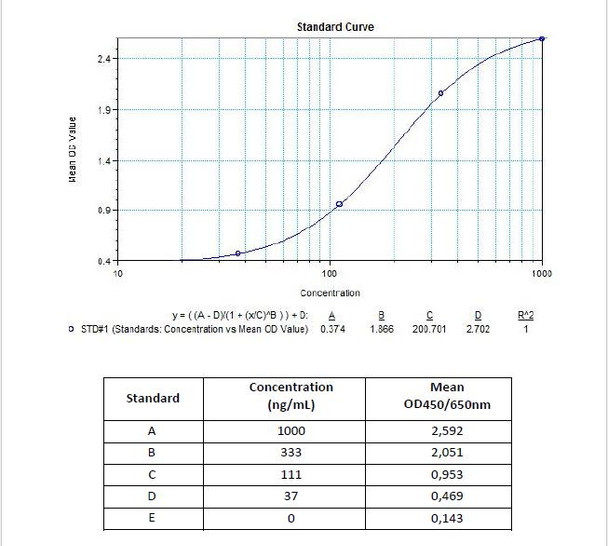
7 x 0.5 mL | Certolizumab pegol Standards A-F, High Level Control, Low Level Control 1000; 333; 111; 37; 0 nanogram/mL |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Certolizumab (Cimzia®) ELISA Protocol
| Steps | Protocol |
1 | Pipette 50µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 50 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
3 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
CIMZIA® is a registered trademark of the UCB Group of Companies.