Therapeutic Drug Monitoring
Canakinumab (Ilaris®)Free drug ELISA Kit
- SKU:
- HUMB00056
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Canakinumab (Ilaris®)
- Research Area:
- Anti-Inflammatory
Description
Assay Genie Canakinumab ELISA has been especially developed for the quantitative analysis of free Canakinumab in serum and plasma samples. Assay Genie Canakinumab ELISA is optimized with Ilaris®.
Canakinumab (Ilaris®) Free drug ELISA Kit Test Principle
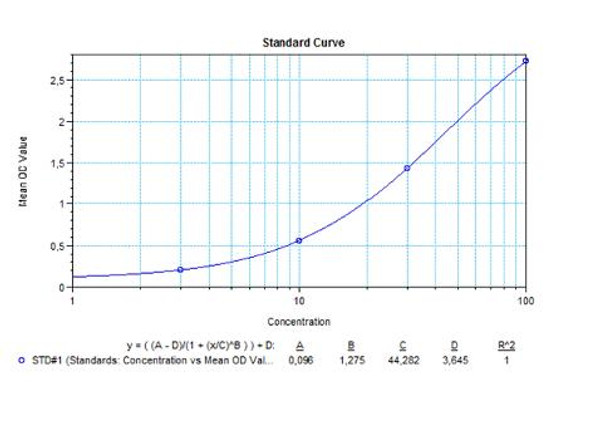
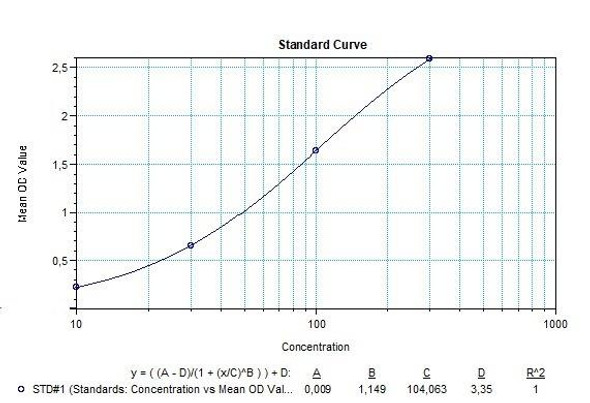
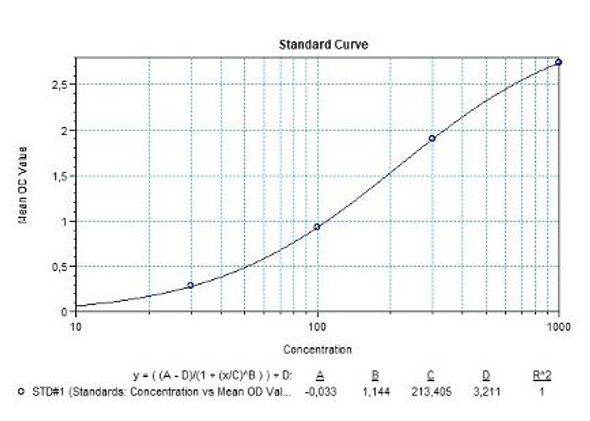
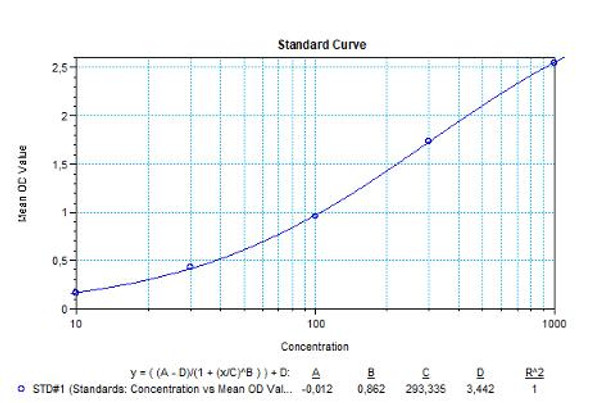
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the reactant for canakinumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to canakinumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of canakinumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Canakinumab (Ilaris®) Free drug ELISA Kit Product Information
| Key Information | Description |
Application | Free Drug |
Required Volume | 10 µl |
Total Time | 70 minutes |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit | 30 ng/mL |
Spike Recovery | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | - |
About Canakinumab (Ilaris®) Free drug ELISA Kit
Canakinumab is a recombinant, human anti-human-IL-1β monoclonal antibody that belongs to the IgG1/κ isotype subclass. Canakinumab binds to human IL-1β and neutralizes its inflammatory activity by blocking its interaction with IL-1
receptors, but it does not bind IL-1alpha or IL-1 receptor antagonist (IL-1ra).
In inflammatory diseases involving Cryopyrin-Associated Periodic Syndromes (CAPS), interleukin-1 beta (IL-1β) is excessively activated and drives inflammation. The protein cryopyrin controls the activation of IL-1β, and mutations in cryopyrin's gene, NLRP-3, up-regulate IL-1β activation. Canakinumab is a human monoclonal anti-human IL-1β antibody of the IgG1/κ isotype. Canakinumab binds to human IL-1β and neutralizes its inflammatory activity by blocking its interaction with IL-1 receptors, but it does not bind IL-1α or IL-1 receptor antagonist (IL-1ra).
Assay Genie ELISA kits can be used for drug level and anti-drug antibodies measurements.
Assay Genie Canakinumab ELISA products: