Therapeutic Drug Monitoring
Bevacizumab (Avastin®) ELISA Kit
- SKU:
- HUMB00024
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Bevacizumab (Avastin®)
- Research Area:
- Anti-Cancer
- Research Area:
- Wet AMD
Description
Bevacizumab (Avastin®) ELISA Kit
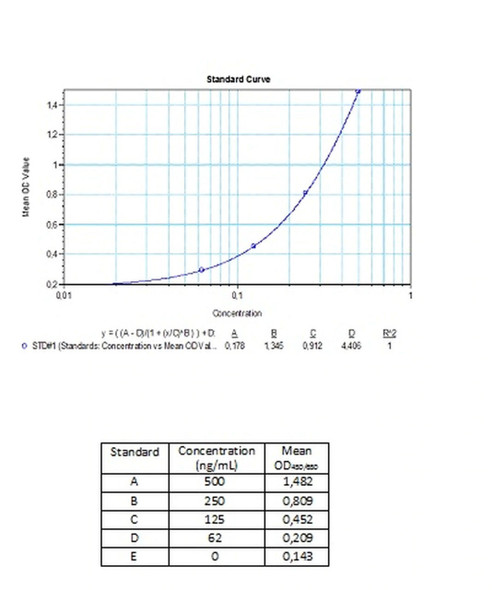
Enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of Bevacizumab (Avastin®) in serum and plasma. The Assay Genie Bevacizumab (Avastin®) ELISA has been developed for the quantitative analysis of the biologically active form of free Bevacizumab (Avastin®) in serum and plasma samples.
Bevacizumab (Avastin®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the double antigen assay principle. Diluted standards and samples (serum or plasma) are incubated in the microtitre plate coated with human vascular endothelial growth factor (VEGF). After incubation, the wells are washed. A biotin conjugated human VEGF is added and binds to bevacizumab (Avastin®) captured by the reactant on the surface of the wells. Following incubation, wells are washed and then HRP conjugated probe (HRP) is added. After incubation, the wells are washed, and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of bevacizumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Bevacizumab (Avastin®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (μl) | 10 |
Total Time (min) | 70 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 30 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-VEGF mAb Avastin |
Bevacizumab (Avastin®) - Key Information
Bevacizumab (Avastin®) mode of action
Bevacizumab (Avastin®) is a recombinant human IgG1:k monoclonal antibody specific for all human vascular endothelial growth factor-A (VEGF-A) isoforms. Bevacizumab carrries out anti-VEGF function by binding circulating VEGF, which in-turn inhibits the binding of VEGF to its cell surface receptors. This inhibition results in a decrease in microvascular growth of tumor blood vessels and therefore limits the blood supply to tumor tissues.
In 1997, the humanization of the murine anti-VEGF mAb was reported. Like its murine counterpart, bevacizumab binds to and neutralizes all human VEGF-A isoforms and bioactive proteolytic fragments, but not mouse or rat VEGF. However, bevacizumab was observed to inhibit the growth of human tumor cell lines in nude mice.
Bevacizumab (Avastin®) uses
The humanized anti-VEGF monoclonal antibody, bevacizumab, has been approved by the FDA as a first-line treatment for metastatic colorectal cancer in combination with chemotherapy. Furthermore, VEGF is implicated in intraocular neovascularization associated with diabetic retinopathy and age-related macular degeneration.
Bevacizumab (Avastin®) history
In 1997, Phase I clinical trials with bevacizumab were initiated. These Phase I studies showed that bevacizumab as a single agent was relatively non-toxic and including standard chemotherapy regimens did not significantly exacerbate chemotherapy associated toxicities.
In 1998, several Phase II studies were initiated with bevacizumab in different tumor types, either as a single agent or in combination with chemotherapy. bevacizumab was combined with Standard first-line chemotherapy in metastatic colorectal cancer and stage IIIb/IV non-small cell lung cancer. The potential clinical utility of VEGF inhibition in oncology is not limited to solid tumors. There is growing evidence that VEGF and VEGF receptors are expressed by a variety of leukemias and other hematologic malignancies. This suggests that inhibition of VEGF or VEGFR signaling may have a role in the treatment of such conditions. Several clinical trials are currently testing these hypotheses.
Although bevacizumab was generally well tolerated, some adverse events were noted. Several open-label Phase I and II clinical trials identified thrombosis and bleeding as potential bevacizumab-related toxicities. Typically, adverse reactions include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain and exfoliative dermatitis.
Bevacizumab (Avastin®) treatment
Studies have demonstrated that bevacizumab, in combination with chemotherapy, resulted in increased survival in patients. These were patients with previously untreated metastatic colorectal cancer. The treatment was compared to chemotherapy as a stand-alone treatment. This lead to FDA approval of the first anti-angiogenic agent.
Bevacizumab (Avastin®) pharmacokinetics
Anti-VEGF monoclonal antibodies and other VEGF inhibitors block the growth of several tumor cell lines in nude mice. Clinical trials with VEGF inhibitors in a variety of malignancies are ongoing. The pharmacokinetic properties of bevacizumab in several species have been previously described and are consistent with a typical humanized monoclonal antibody.
Bevacizumab is administered up to 15 mg/kg (Non- squamous non-small cell lung cancer: 15 mg/kg IV every 3 weeks with carboplatin/paclitaxel) in patients without evidence of dose limiting toxicities.
Bevacizumab (Avastin®) ELISA Kit Contents
| Size | Kit Contents |
1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
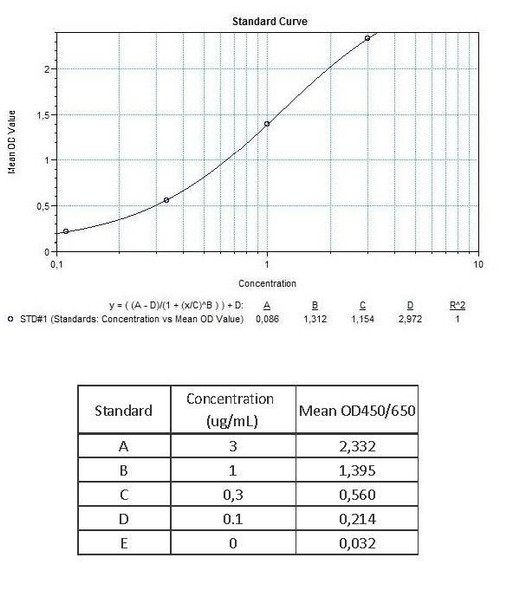
7 x 0.3 mL | Bevacizumab Standards A-E (100X), 100; 30; 10; 3; 0 µg/mL |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Bevacizumab (Avastin®) ELISA Protocol
| Steps | Protocol |
1 | Dilute each of the standards and samples (serum/plasma) at 1:100 using Assay Buffer as described in “Dilution of Standards and Samples (serum/plasma)” section of the technical manual. |
2 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
3 | Pipette 25 µL of Diluted Standards, High Level Control, Low Level control and Samples into the respective wells of microtiter plate. Wells |
4 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
5 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
6 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
7 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
8 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
9 | Pipette 100 µL of TMB Substrate Solution into each well. |
10 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
11 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
12 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Avastin® is a registered trademark of Genentech, Inc.