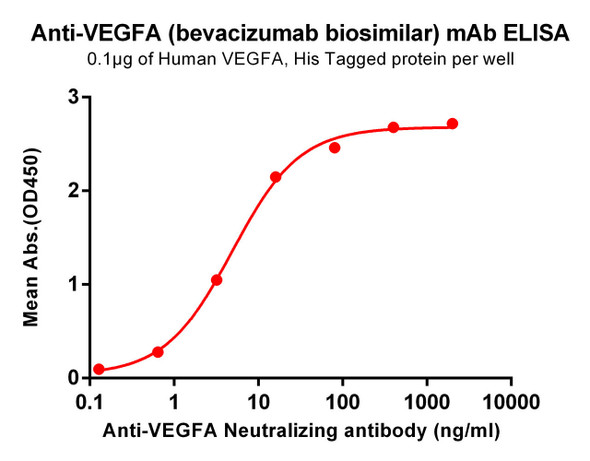
Description
system_update_altDatasheet
| Product Name: | Bevacizumab (Anti-VEGF) Biosimilar In Vivo Antibody |
| Product Code: | IVMB0232 |
| Clone: | A4.6.1 |
| Protein: | VEGF |
| Product Type: | Biosimilar Recombinant Human Monoclonal Antibody |
| Synonyms: | Vascular Endothelial Growth Factor, VEGF-A, VEGFA, Vascular Permeability Factor, VPF |
| Isotype: | Human IgG1κ |
| Reactivity: | Human |
| Immunogen: | Recombinant human VEGF. |
| Applications: | B, ELISA, FC, IP, N, WB |
| Formulation: | This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. |
| Endotoxin Level: | < 1.0 EU/mg as determined by the LAL method |
| Purity: | ≥95% monomer by analytical SEC |
| Preparation: | Functional grade preclinical antibodies are manufactured in an animal free facility using only In vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. |
| Storage and Handling: | Functional grade biosimilar antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at -80°C. Avoid Repeated Freeze Thaw Cycles. |
| Applications: | B, ELISA, FC, IP, N, WB |
| Recommended Usage: | FC The suggested concentration for Adalimumab biosimilar antibody for staining cells in flow cytometry is ≤ 0.25 µg per 106 in a volume of 100 µl. Titration of the reagent is recommended for optimal performance for each application. WB ELISA |
| Reactivity: | Human |
| Specificity: | This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Bevacizumab. Bevacizumab recognizes both native and reduced human VEGF (isoform 165). This product is for research use only. |
| Antigen Distribution: | VEGF is widely expressed in the thyroid, prostate, and various other tissues. |
| Immunogen: | Recombinant human VEGF. |
| Concentration: | ≥ 5.0 mg/ml |
| Endotoxin Level: | < 1.0 EU/mg as determined by the LAL method |
| Purity: | ≥95% monomer by analytical SEC |
| Formulation: | This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. |
| Preparation: | Functional grade preclinical antibodies are manufactured in an animal free facility using only In vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. |
| Storage and Handling: | Functional grade biosimilar antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at -80°C. Avoid Repeated Freeze Thaw Cycles. |
| Protein: | VEGF |