Therapeutic Drug Monitoring
Avelumab (Bavencio®) ELISA Kit
- SKU:
- HUMB00042
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Avelumab (Bavencio®)
- Research Area:
- Checkpoint Inhibitors
Description
Avelumab (Bavencio®) ELISA Kit
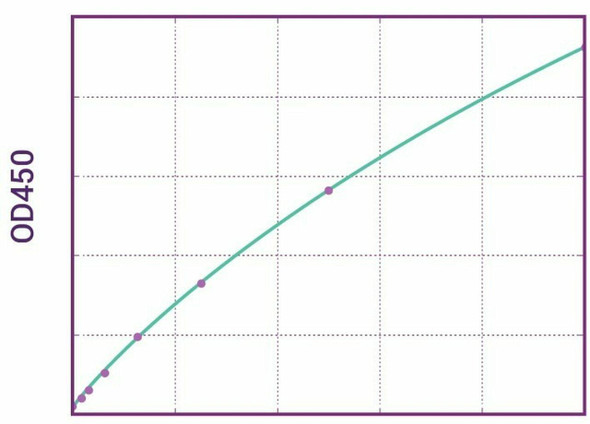
Enzyme-linked immunosorbent assay for the quantitative determination of free Avelumab (Bavencio®) in serum and plasma. The Assay Genie Avelumab (Bavencio®) ELISA has been especially developed for the quantitative analysis of free avelumab in serum and plasma samples.
Avelumab (Bavencio®) ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtitre plate coated with the reactant for Avelumab (Bavencio®). After incubation, the wells are washed. A horse radish peroxidase (HRP) conjugated probe is added and binds to avelumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of avelumab in the sample or standard. Results of samples can be determined directly using the standard curve
Avelumab (Bavencio®) ELISA Product Information
| Information | Description |
| Application | Free drug |
| Required Volume (uL) | 10 |
| Total Time (min) | 70 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | 10 (ng/mL) |
| Spike Recovery (%) | 85-115% |
| Shelf Life (year) | 1 |
| Alternative Names | Anti-PD-L1 IgG1 lambda mAb Bavencio |
Avelumab (Bavencio®) - Key Information
Avelumab (Bavencio®) mode of action
Avelumab (Bavencio®) is an investigational fully human anti-PD-L1 IgG1 lambda monoclonal antibody that has a molecular weight of approximately 147 kDa. By inhibiting PD-L1 interactions, Avelumab is thought to enable the activation of T-cells and the adaptive immune system. By retaining a native Fc-region, avelumab is thought to engage the innate immune system and may induce antibody-dependent cellmediated cytotoxicity (ADCC). Importantly, avelumab has not shown antibody-dependent cell-mediated cytotoxicity against immune cell subsets in humans.
Avelumab (Bavencio®) uses
Avelumab is a programmed death ligand-1 (PD-L1) blocking antibody. It is used for the treatment of adults and pediatric patients greater than 12 years with metastatic Merkel Cell Carcinoma (MCC).
Avelumab (Bavencio®) and PD-L1
The mechanism of Action PD-L1 may be expressed on tumor cells and tumor-infiltrating immune cells and can contribute to the inhibition of the anti-tumor immune response in the tumor microenvironment. Binding of PD-L1 to the PD-1 and B7 receptors found on T cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation and cytokine production. Avelumab binds PD-L1 and blocks the interaction between PD-L1 and its receptors PD1 and B7.1. This interaction releases the inhibitory effects of PD-L1 on the immune response resulting in the restoration of immune responses, including anti-tumor immune responses.
Avelumab has also been shown to induce antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro. In syngeneic mouse tumor models, blocking PD-L1 activity resulted in decreased tumor growth.
Avelumab (Bavencio®) pharmacokinetics
The pharmacokinetics of Avelumab was studied in 1629 patients who received doses ranging from 1 to 20 mg/kg every 2 weeks. The data showed that the exposure of Avelumab increased dose-proportionally in the dose range of 10 to 20 mg/kg every 2 weeks. Steady-state concentrations of Avelumab were reached after approximately 4 to 6 weeks (2 to 3 cycles) of repeated dosing, and the systemic accumulation was approximately 1.25-fold. According to FDA's data, Cmax value was found 30 μg/mL and Cmin value was found as 22 μg/mL. In observed ADA-positive patients, the Cmin value was increased to 27.2 μg/mL, while in ADA-negative patients, this value decreased to 14.1 μg/mL. Distribution The geometric mean volume of distribution at steady state for a subject receiving 10 mg/kg was 4.72 L
The primary elimination mechanism of Avelumab is proteolytic degradation. Based on population pharmacokinetic analyses in patients with solid tumors, the total systemic clearance was 0.59 L/day and the terminal half-life was 6.1 days in patients receiving 10 mg/kg. In a post hoc analysis, avelumab clearance was found to decrease over time in patients with MCC, with a mean maximal reduction (% coefficient of variation [CV%]) from baseline value of approximately 41.7% (40.0%). Body weight was positively correlated with total systemic clearance in population pharmacokinetic analyses.
Avelumab (Bavencio®) ELISA Kit Contents
| Size | Kit Contents |
| 1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
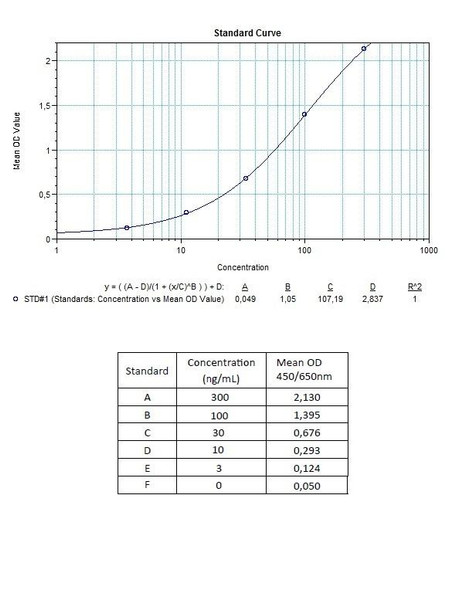
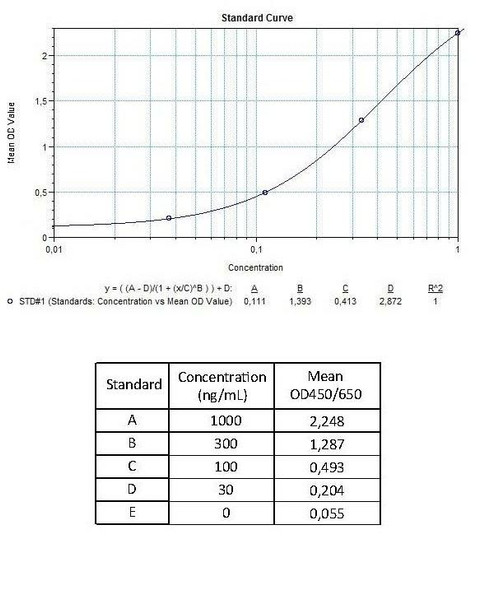
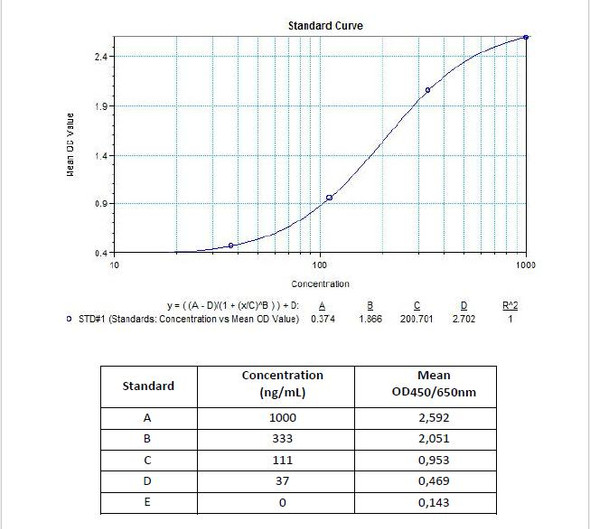
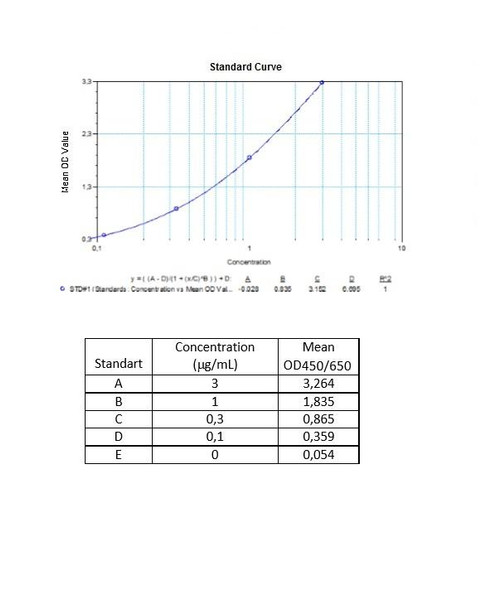
| 7 x 0.1 mL | Avelumab Standards A-E (100x), High Level Control, Low Level Control 300; 100; 30; 10; 0 microgram/mL. Used for construction of the standard |
| 1 x 50 mL | Assay Buffer |
| 1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
Avelumab (Bavencio®) ELISA Protocol
| Steps | Protocol |
| 1 | Dilute each of the standards and samples (serum/plasma) at 1:100 using Assay Buffer as described in “Dilution of Standards and Samples (standards/serum/plasma)”section of the techical manual. |
| 2 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
| 3 | Pipette 10 µL of each Diluted Standards, Diluted High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
| 4 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
| 5 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 6 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
| 7 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
| 8 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 9 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 10 | Incubate 10 min (without adhesive foil.) at room temperature (18-25°C) in the dark. |
| 11 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 12 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
BAVENCIO is a trademark of MERCK KGAA