Therapeutic Drug Monitoring
Anti-Ustekinumab (Stelara®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00021
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Ustekinumab (Stelara®-
- Research Area:
- Anti-Inflammatory
Description
Anti-Ustekinumab (Stelara®) ADA Qualitative ELISA Kit
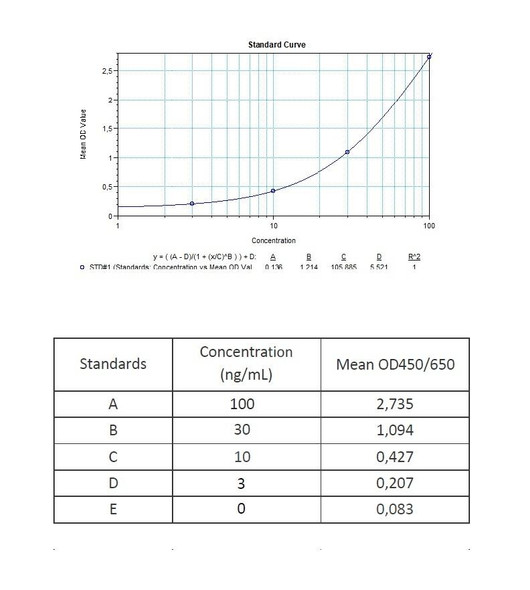
Enzyme-linked immunosorbent assay (ELISA) for the qualitative determination of specific anti-Ustekinumab (Stelara®) antibodies in human serum and plasma. The Assay Genie Antibody to Ustekinumab (Stelara®) ELISA Kit is intended for the qualitative determination of Ustekinumab Anti-Drug Antibodies (ADA) in serum and plasma. It is for professional use only.
Anti-Ustekinumab (Stelara®) ADA Qualitative ELISA Kit test principle
The Assay Genie Antibody to Ustekinumab (Stelara®) ELISA is a sandwich assay for the determination of antibodies against Ustekinumab in serum and plasma samples. During the first incubation period, anti-Ustekinumab antibodies in patient serum/ plasma samples are captured by the Ustekinumab (Stelara®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of Ustekinumab in sample.
Anti-Ustekinumab (Stelara®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (µL) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | plus/minus |
Spike Recovery (%) | - |
Shelf Life (year) | 1 |
Alternative Names | Stelara |
Anti-Ustekinumab ADA Qualitative ELISA - Key Information
Ustekinumab (Stelara®) mode of action
Ustekinumab is a fully human monoclonal antibody that binds with high specificity and affinity to the cytokines interleukin (IL)-12 and IL-23, thereby suppressing IL-12- and IL-23-mediated inflammation associated with psoriasis. Ustekinumab is a human IgG1-kappa monoclonal antibody designed to interfere with the triggering of the body's inflammatory response through the suppression of certain cytokines. Specifically, ustekinumab blocks interleukin IL-12 and IL-23 by binding with high affinity and specificity to the p40 subunit of IL-12 and IL-23, therefore disrupting the proinflammatory pathway that contributes to several chronic diseases. In a more minor role, ustekinumab also interferes with the expression of monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-alpha), interferon-inducible protein-10 (IP-10), and interleukin IL-8.
Ustekinumab (Stelara®) uses
Ustekinumab is used as a treatment for plaque psoriasis and a particular type of arthritis (psoriatic arthritis). Furthermore, ustekinumab is used for certain bowel conditions including Crohn's disease and ulcerative colitis. Ustekinumab (STELARA®) is indicated for the treatment of adult patients with moderate to severe plaque psoriasis (Ps) who are candidates for phototherapy or systemic therapy. It works by blocking certain natural proteins in your body (interleukin-12 and interleukin-23) that cause inflammation (swelling) in these conditions. Ustekinumab (STELARA®) is the only FDA-approved medicine that targets IL-12 and IL-23, which are thought to be associated with gastrointestinal inflammation in Crohn's disease.
Ustekinumab (Stelara®) immunogenicity
As with any biologic therapy, immunogenicity, in the form of anti-ustekinumab antibodies, occurs with following administration. The demonstration of anti-ustekinumab antibodies during treatment with ustekinumab (Stelara®) is a major concern. Monitoring for the presence and/or quantitation of specific antibodies during clinical trials is an important issue for treatment regimen follow-up. The Assay Genie Anti-Ustekinumab ADA Qualitative ELISA Kit can be efficiently used for monitoring ustekinumab-specific antibodies in biological samples and are for research use only. With this Assay Genie ELISA test, ustekinumab anti-drug antibodies (ADA) can be detected.
Ustekinumab (Stelara®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
7 x 0.3 mL | Reactive Control |
1 x 50 mL | Negative Control |
1 x 12 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Ustekinumab (Stelara®) ELISA Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | QUALITATIVE ELISA TEST FORMAT Wells |
3 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Stelara® is a registered trademark of Johnson and Johnson, Inc.