Therapeutic Drug Monitoring
Anti-Trastuzumab (Herceptin®, Herclon®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00031
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Trastuzumab (Herceptin®/Herclon®)
- Research Area:
- Anti-Cancer
Description
Anti-Trastuzumab ADA Qualitative ELISA Kit
Enzyme-linked immunosorbent assay (ELISA) for the qualitative determination of antibodies to Trastuzumab (Herceptin®, Herclon®) in serum and plasma.The ELISA Genie Anti-Trastuzumab (Herceptin®, Herclon®) ELISA Kit is intended for the qualitative determination of Trastuzumab Anti-Drug Antibodies (ADA) in serum and plasma.
Anti-Trastuzumab (Herceptin®, Herclon®) ADA Qualitative ELISA Kit test principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Controls and samples (serum or plasma) are incubated in the microtiter plate coated with the drug trastuzumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to trastuzumab antibodies captured by the drug trastuzumab on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of trastuzumab antibodies in the sample or controls. The results can be evaluated with using cut-off value.
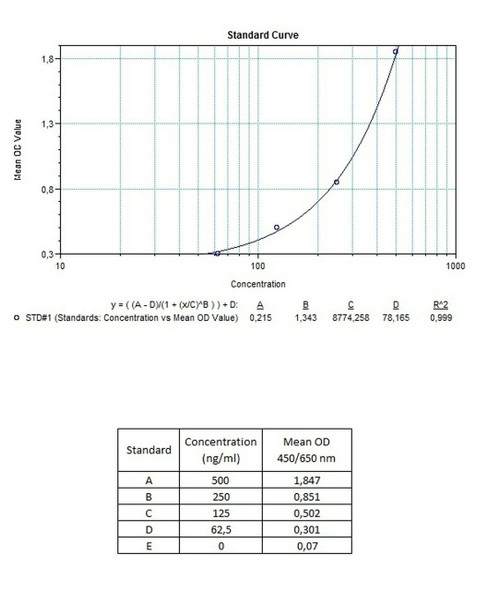
Anti-Trastuzumab (Herceptin®, Herclon®) ADA Qualitative ELISA Product Information
| Information | Description |
Application | Free drug |
Required Volume (μl) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 30 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Human Epidermal Growth Factor Receptor 2 (HER2) |
Anti-Trastuzumab ADA Qualitative ELISA - Key Information
Trastuzumab (Herceptin®, Herclon®) mode of action
Trastuzumab (Herclon®, Herceptin®) is a recombinant DNA-derived humanized monoclonal antibody that selectively targets the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2). The antibody is an IgG1 kappa that contains human framework regions with the complementarity-determining regions of a murine anti-p185 HER2 antibody that binds to HER2.
Trastuzumab is composed of 1,328 amino acids and has a molecular weight of 148 kDa. Trastuzumab has anti-tumor activity against HER2-positive human breast tumor cells in laboratory models and is active for the treatment of women with HER2-overexpressing breast cancers. On the basis of trastuzumab clinical efficacy, this antibody was approved in 1998 for clinical use for HER2 overexpressing metastatic breast cancer.
Trastuzumab exerts its anti-tumor effects by several mechanisms that are not yet completely understood. In HER2 overexpressing cells, trastuzumab markedly down-regulates HER2 expression by accelerating receptor endocytosis and degradation and inhibits cell cycle progression by inducing the formation of p27Kip1/Cdk2 complexes. Trastuzumab also induces antibody-dependent cell-mediated cytotoxicity against the HER2 expressing tumor cells in animal models. This process is regulated by antibody receptors FcγRIII and FcγRIIB on myeloid cells. Other additional mechanisms that have been proposed include suppression by trastuzumab of angiogenesis and metastasis.
Trastuzumab (Herceptin®, Herclon®) uses
Trastuzumab is used to treat metastatic breast cancer and stomach cancer. It is effective against tumors that possess HER2/neu protein overexpression. Traztuzumab can be used alone or in combination with other chemotherapeutic medication. Traztuzumab is used in cancers that are HER2 receptor positive.
Trastuzumab (Herceptin®, Herclon®) and the HER2/neu protein
The HER (or ErbB) family of transmembrane tyrosine kinase receptors is composed of four members, HER1 to HER4 . HER2, a ligand-less Mr (molecular mass of the repeat unit) 185,000 receptor encoded by the neu proto-oncogene, is overexpressed in 25-30% of human breast cancer and has been associated with enhanced tumor aggressiveness and a high risk of relapse and death.
Recent evidence indicates that HER2 amplifies the signal provided by other receptors of the ErbB family by heterodimerizing with them. The important biological role of HER2 in the signaling network that drives epithelial cell proliferation and transformation, together with its extracellular accessibility and its overexpression in some human tumors led to considering HER2 as an appropriate target for tumor-specific therapies.
The neu gene encodes a 185-kDa transmembrane glycoprotein, referred to as p185neu, HER2, or erbB-2, possessing intrinsic protein tyrosine kinase activity. The receptor consists of an extracellular domain, with four subdomains including two cysteine rich domains, a transmembrane domain, and an intracellular domain, consisting of a juxtamembrane region, a tyrosine kinase domain, and a carboxyl tail harboring autophosphorylation sites HER2 is homologous to, but distinct from, other members of the erbB family, which includes the epidermal growth factor receptor (EGFR or erbB-1), erbB-3, and erbB-4.
The binding of cognate growth factors to these receptors regulates cell growth, proliferation, and diferentiation through the activation of receptor tyrosine kinases, triggering an incompletely defined signal transduction cascade. Signal transduction by these receptors is believed to involve dimerization and oligomerization, both in the form of homo-oligomers and hetero-oligomers in various erbB receptor combinations.
Trastuzumab (Herceptin®, Herclon®) immunogenicity
When administered to patients, all therapeutic proteins have the potential to induce an unwanted immune response, for example, to stimulate the formation of anti-drug antibodies (ADAs). The impact of immune responses can range from no apparent effect to changes in pharmacokinetics to loss of effect and serious adverse events. This ELISA Genie Anti-Trastuzumab (Herceptin®, Herclon®) ELISA can be used for the qualitative determination of anti-Trastuzumab antibodies in serum and plasma and is for research use only.
Anti-Trastuzumab (Herceptin®, Herclon®) ADA Qualitative ELISA Kit Contents
| Size | Kit Contents |
1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
1.0 mL (negative) | Control negative and positive |
1 x 12 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Anti-Trastuzumab (Herceptin®, Herclon®) ADA Qualitative ELISA Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 10 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
3 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Herceptin® is a registered trademark of Genentech, Inc.