Therapeutic Drug Monitoring
Anti-Rituximab (Rituxan®, Mabthera®)ADA Quantitative ELISA Kit
- SKU:
- HUMB00029
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Rituximab (Rituxan®/Mabthera®)
- Research Area:
- Anti-Cancer
Description
Anti-Rituximab ADA Quantitative ELISA Kit
Enzyme-linked immunosorbent assay for the quantitative determination of antibodies to Rituximab (Rituxan®, Mabthera®) in serum and plasma. The Assay Genie anti-Rituximab ELISA Kit is intended for the quantitative determination of rituximab anti-drug antibodies (ADA) in serum and plasma and is for research use only.
Anti-Rituximab (Rituxan®, Mabthera®) ADA Quantitative ELISA Kit test principle
The Assay Genie Antibody to Rituximab (Rituxan®, Mabthera®) ELISA is a double-antigen sandwich assay for the determination of antibodies against rituximab in serum and plasma samples. During the first incubation period, the drug rituximab coated on the wall of the microtiter wells captures the antibodies to rituximab in patient serum and plasma samples. After washing away the unbound components from samples, a Peroxidase-rituximab conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of antibodies to rituximab in sample.
Anti-Rituximab (Rituxan®, Mabthera®) ADA Quantitative Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
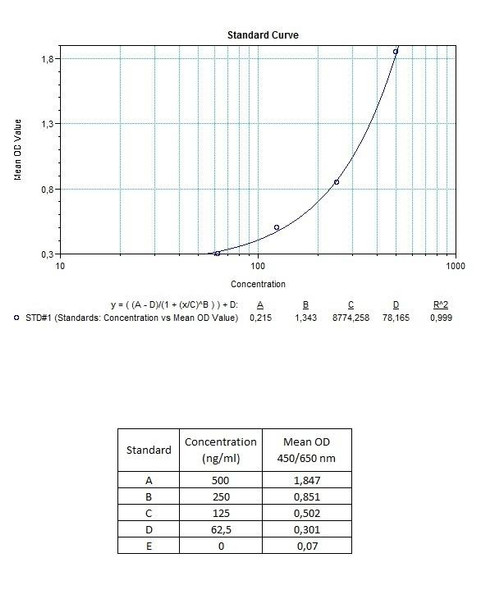
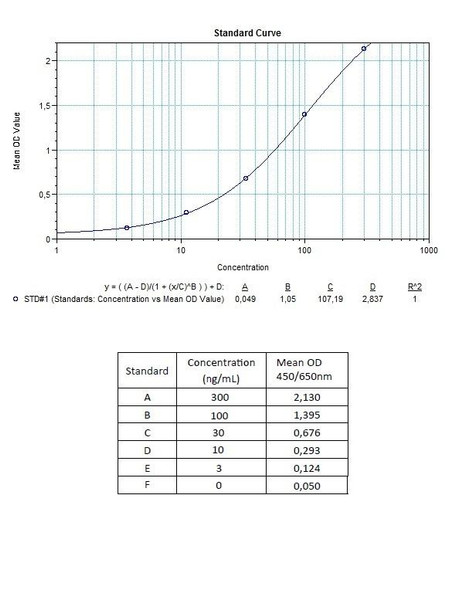
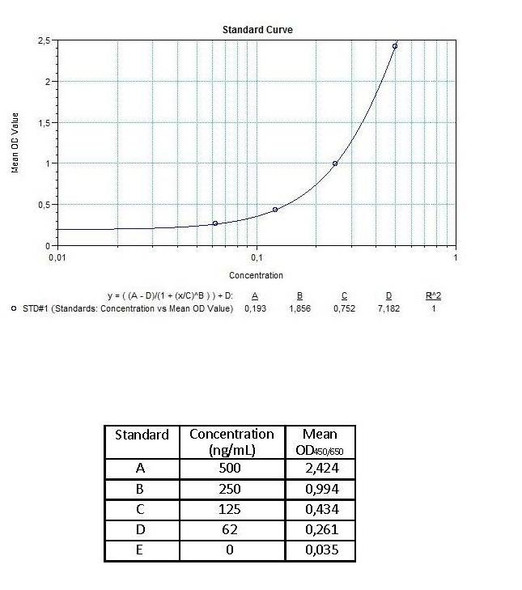
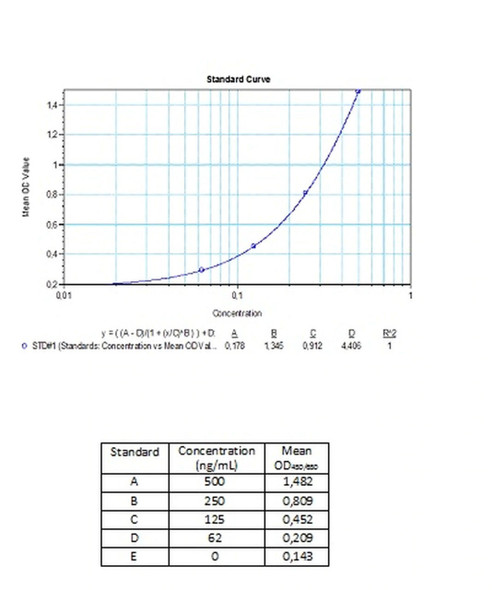
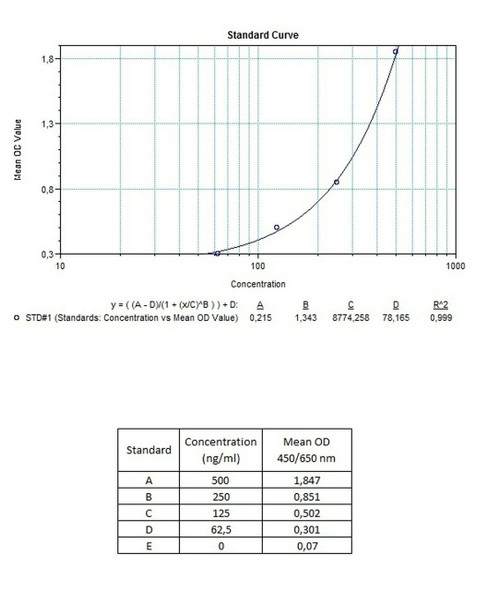
Detection Limit (ng/mL) | 30 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Anti-CD20 mAb Rituxan |
Anti-Rituximab ADA Quantitative ELISA - Key Information
Rituximab (Rituxan®, Mabthera®) mode of action
Rituximab is a genetically engineered chimeric murine/human monoclonal antibody directed against the CD20 antigen found on the surface of normal and malignant B lymphocytes. The antibody is a glycosylated IgG1 kappa immunoglobulin containing murine light- and heavy-chain variable region sequences (Fab domain) and human constant region sequences (Fc domain). Rituximab is composed of 1,328 amino acids and has an approximate molecular weight of 144 kD. Rituximab has a high binding affinity for the CD20 antigen of 5.2 to 11.0 nM. Rituximab binds specifically to the antigen CD20, a transmembrane molecule located on pre-B and mature B lymphocytes.
Rituximab (Rituxan®, Mabthera®) uses
Rituximab is a CD20-directed cytolytic antibody indicated for the treatment of patients with: Non-Hodgkin's Lymphoma (NHL), Chronic Lymphocytic Leukemia, Rheumatoid Arthritis (RA) in combination with methotrexate in adult patients with moderately-to severely-active RA who have inadequate response to one or more TNF antagonist therapies, Wegener's Granulomatosis (WG) and Microscopic Polyangiitis (MPA) in adult patients in combination with glucocorticoids.
Rituximab (Rituxan®, Mabthera®) treatment
The CD20 antigen is expressed on > 95% of all B-cell non-Hodgkin's lymphomas (NHL). CD20 (human B lymphocyte-restricted differentiation antigen, Bp35) is a hydrophobic transmembrane protein with a molecular weight of approximately 35 kD. This non-glycosylated phosphoprotein is found on both normal and malignant B cells, but not on haematopoietic stem cells, pro-B cells, normal plasma cells or other normal tissues. CD20 regulates (an) early step(s) in the activation process for cell cycle initiation and differentiation, and possibly functions as a calcium ion channel. CD20 does not internalise upon antibody binding and is not shed from the cell surface. This antigen does not circulate in the plasma. Thus, free antigen does not compete for rituximab binding.
The Fab domain of rituximab binds to the CD20 antigen on B-lymphocytes and the Fc domain recruits immune effector functions to mediate B-cell lysis. Possible mechanisms of cell lysis include complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). The antibody also induces apoptosis in the DHL-4 human B-cell lymphoma line and in vitro studies have demonstrated that rituximab sensitises drug resistant human B-cell lymphoma lines to the cytotoxic effects of some chemotherapeutic agents. In human tissue, the expression of the CD20 antigen is highly restricted; rituximab binding to CD20 was found only on lymphoid cells in the thymus, the white pulp of the spleen and a majority of B lymphocytes in peripheral blood and lymph nodes. Little or no non-specific binding was observed.
Rituximab (Rituxan®, Mabthera®) immunogenicity
As with any biologic therapeutic, immunogenicity, in the form of anti-rituximab antibodies, occurs with following administration. The use of rituximab (Rituxan®, Mabthera®) was associated with the development of anti-rituximab antibodies, with some having neutralizing effects, in a number of patients during therapy with the drug. The Assay Genie Anti-Rituximab ELISA Kits can be used to detect and measure the presence of rituximab anti-drug antibodies (ADA) in biological samples and is for research use only.
Anti-Rituximab ADA Quantitative ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
7 x 1 mL | ATR Standards A-E, High Level Control, Low Level Control |
1 x 12 mL | Confirmation Reagent |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Anti-Rituximab ADA Quantitative ELISA Protocol
| Steps | Protocol |
1 | QUANTITATIVE ELISA TEST FORMAT Wells |
2 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
3 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
4 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
5 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
6 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
7 | Pipette 100 µL of TMB Substrate Solution into each well. |
8 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
9 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
10 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Rituxan® is a registered trademark of Biogen Idec, Inc.