Therapeutic Drug Monitoring
Anti-Ramucirumab (Cyramza®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00023
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Ramucirumab (Cyramza®)
- Research Area:
- Anti-Cancer
Description
Anti-Ramucirumab ADA Qualitative ELISA Kit
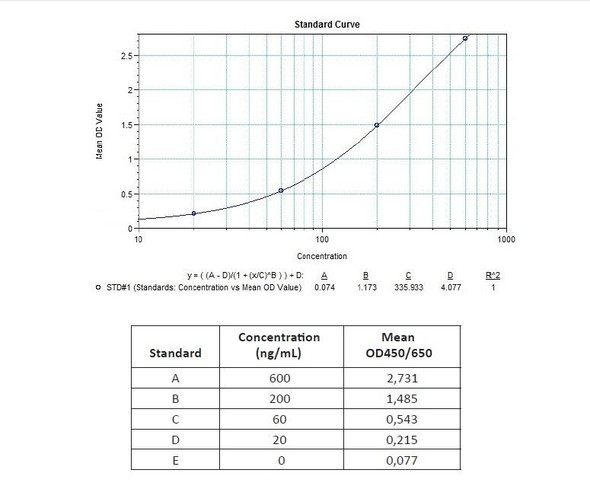
Enzyme-linked immunosorbent assay for the qualitative determination of anti-Ramucirumab antibodies in serum and plasma.The Assay Genie Antibody to Ramucirumab (Cyramza®) ELISA Kit is intended for the qualitative determination of Ramucirumab anti-drug antibodies (ADA) in serum and plasma.
Anti-Ramucirumab (Cyramza®) ADA Qualitative ELISA Kit test principle
The Assay Genie Antibody to Ramucirumab (Cyramza®) ELISA is a sandwich assay for the determination of antibodies against ramucirumab in serum and plasma samples. During the first incubation period, the drug ramucirumab coated on the wall of the microtiter wells captures the antibodies to ramucirumab in patient serum and plasma samples. After washing away the unbound components from samples, a Peroxidase-labelled conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of antibodies to Ramucirumab in sample.
Anti-Ramucirumab (Cyramza®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 140 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | plus/minus |
Spike Recovery (%) | - |
Shelf Life (year) | 1 |
Alternative Names | Anti-VEGFR2 mAb Cyramza |
Anti-Ramucirumab ADA Qualitative - Key Information
Ramucirumab (Cyramza®) mode of action
Ramucirumab is a human monoclonal antibody (IgG1) against vascular endothelial growth factor receptor 2 (VEGFR2), a type II trans-membrane tyrosine kinase receptor expressed on endothelial cells. By binding to VEGFR2, ramucirumab prevents binding of its ligands (VEGF-A, VEGF-C, and VEGF-D), thereby preventing VEGF-stimulated receptor phosphorylation and downstream ligand-induced proliferation, permeability, and migration of human endothelial cells. VEGFR stimulation also mediates downstream signalling required for angiogenesis and is postulated to be heavily involved in cancer progression, making it a highly likely drug target. In contrast to other agents directed against VEGFR-2, ramucirumab binds a specific epitope on the extracellular domain of VEGFR-2, thereby blocking all VEGF ligands from binding to it.
Ramucirumab (Cyramza®) uses
Ramucirumab (Cyramza®) is used to treat stomach (gastric) cancer, colorectal cancer, Hepatocellular carcinoma or non-small cell lung cancer that has metastasized to other parts of the body. Ramucirumab (Cyramza®) may be given alone or in combination with other cancer medicines.
Ramucirumab is indicated for use in advanced gastric or gastro-esophageal junction adenocarcinoma as a single agent or in combination with paclitaxel after prior fluoropyrimidine- or platinum-containing chemotherapy. According to the information given by the manufacturer; Cmax: 171 µg/ml, Cmin: 49.5 µg/ml. Ramucirumab packaging includes warnings for arterial thromboembolic events, hypertension, infusion-related reactions, gastrointestinal perforation, clinical deterioration in patients with cirrhosis, and reversible posterior leukoencephalopathy syndrome.
The most common reactions observed in single-agent-treated patients at a rate of >10% and >2% higher than placebo were hypertension and diarrhea. The most common adverse reactions observed in patients treated with ramucirumab plus paclitaxel at a rate of >30% and >2% higher than placebo plus paclitaxel were fatigue, neutropenia, diarrhea, and epistaxis.
Ramucirumab (Cyramza®) immunogenicity
As with any biologic therapy, immunogenicity, in the form of anti-ramucirumab antibodies, occurs with following administration. The demonstration of anti-ramucirumab antibodies during treatment with ramucirumab (Cyramza®) is a major concern. Monitoring for the presence and/or quantitation of specific antibodies during clinical trials is an important issue for treatment regimen follow-up. The Assay Genie Anti-ramucirumab ADA Qualitative ELISA Kit can be efficiently used for monitoring ramucirumab-specific antibodies during therapy and offers the clinician a tool for a decision on possible preventive measures. This includes the potential addition of an immunosuppressive drug to reduce anti-ramucirumab antibodies. With this Assay Genie ELISA test, antibodies to ramucirumab can be detected in patients receiving Cyramza®.
Anti-Ramucirumab (Cyramza®) ADA Qualitative ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
1 mL (positive) | Control negative and positive |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Anti-Ramucirumab (Cyramza®) ADA Qualitative ELISA Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 10 µL of each “Negative control”, “Positive control” and samples into the Wells *It is advised to run more than one “Negative control” samples. Negative control |
3 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use Conjugate into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
CYRAMZA® is a trademark owned by or licensed to Eli Lilly and Company , its subsidiaries, or affiliates.