Therapeutic Drug Monitoring
Anti-Pembrolizumab (Keytruda®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00047
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Pembrolizumab (Keytruda®)
- Research Area:
- Checkpoint Inhibitors
Description
Anti-Pembrolizumab ADA Qualitative ELISA Kit
Enzyme-linked immunosorbent assay for the qualitative determination (screening) of antibodies to Pembrolizumab (Keytruda®) in serum and plasma. The Assay Genie Antibody to Pembrolizumab (Keytruda®) ELISA Kit is intended for the qualitative determination of antibodies to pembrolizumab (Keytruda®) in serum and plasma. It is for professional use only.
Anti-Pembrolizumab (Keytruda®) ADA Qualitative ELISA Kit test principle
The Assay Genie Antibody to Pembrolizumab (Keytruda®) ELISA is a sandwich assay for the determination of antibodies against Pembrolizumab (anti-pembrolizumab antibodies) in serum and plasma samples. During the first incubation period, antibodies to Pembrolizumab (ATP) in patient serum/ plasma samples are captured by the drug Pembrolizumab (Keytruda®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase-labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-pembrolizumab antibodies in sample.
Anti-Pembrolizumab (Keytruda®) ADA Qualitative ELISA Product Information
| Information | Description |
| Application | Free drug |
| Required Volume (uL) | 10 |
| Total Time (min) | 140 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
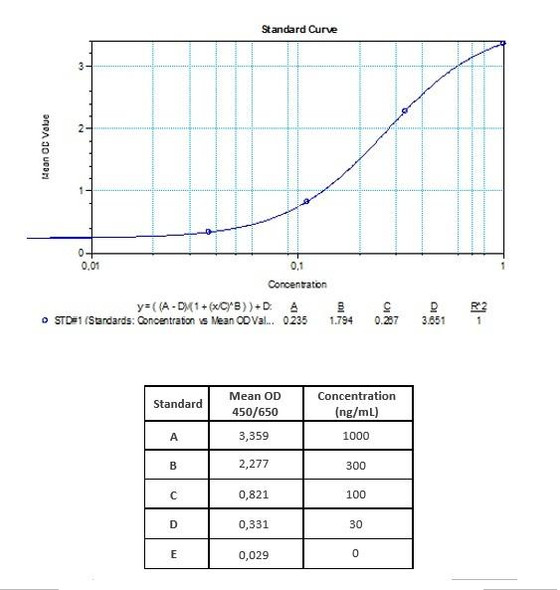
| Detection Limit (ng/mL) | plus/minus |
| Spike Recovery (%) | - |
| Shelf Life (year) | 1 |
| Alternative Names | Keytruda |
Anti-Pembrolizumab (Keytruda®) ADA Qualitative - Key Information
Pembrolizumab (Keytruda®) mode of action
Pembrolizumab (Keytruda®) is a humanized monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. When pembrolizumab (Keytruda®) binds to the PD-1 receptor and blocks both immune-suppressing ligands, PDL1 and PDL2, it helps to restore T-cell response and immune response.
Pembrolizumab (Keytruda®) uses
Pembrolizumab (Keytruda®) is used for the treatment of several types of cancer such as, Melanoma, Non-Small Cell Lung Cancer and Head and Neck Cancer. Due to its success in clinical trials, Pembrolizumab was approved early to allow quick patient access and was given breakthrough therapy and orphan drug designation. Pembrolizumab (as Keytruda) was approved by the U.S. Food and Drug Administration to treat advanced cases of the most common type of lung malignancy, non-small cell lung cancer on Oct. 2, 2015. Keytruda was additionally approved for the treatment of Classical Hodgkin Lymphoma (cHL) in March 2017 Pembrolizumab is a programmed death receptor-1 (PD-1)-blocking antibody indicated for the treatment of the patients with unresectable or metastatic melanoma.
Pembrolizumab (Keytruda®) treatment
Upregulation of PD-1 ligands is a mechanism for tumours to evade antitumor immune response. When PD-1 binds its ligand, the T cell receives an inhibitory signal, which leads to T cell anergy and blockade of anti-tumour immune response. Instead of directly targeting tumor tissue to induce tumor cell death, Pembrolizumab acts as a checkpoint inhibitor to stimulate immune responses to eliminate cancer cells.
Steady-state concentrations of pembrolizumab were reached by 19 weeks of repeated dosing with an every 3- week regimen and the systemic accumulation was 2.2-fold. The peak concentration (Cmax), trough concentration (Cmin), and area under the plasma concentration versus time curve at steady state (AUCss) of pembrolizumab increased dose proportionally in the dose range of 2 to 10 mg/kg every 3 weeks.
Pembrolizumab (Keytruda®) immunogenicity
As with any biologic therapeutic, immunogenicity, in the form of anti-pembrolizumab antibodies can occur. The demonstration of anti-pembrolizumab antibodies during treatment with pembrolizumab is a major concern. The Assay Genie Anti-Pembrolizumab ADA Qualitative ELISA Kit can be efficiently used for monitoring anti-pembrolizumab antibodies in biological samples and is for research use only.
Anti-Pembrolizumab (Keytruda®) ADA Qualitative ELISA Kit Contents
| Size | Kit Contents |
| 1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
| 1 x 0.25 mL | Reactive Control |
| 1 x 0.5 mL | Negative Control |
| 1 x 12 mL | Assay Buffer |
| 1 x 12 mL | Peroxidase Conjugate |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
Anti-Pembrolizumab (Keytruda®) ADA Qualitative ELISA Protocol
| Steps | Protocol |
| 1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
| 2 | QUALITATIVE ELISA TEST FORMAT Wells |
| 3 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 5 | Pipette 100 µL of ready-to use Peroxidase into each well. |
| 6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 8 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
| 10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Keytruda® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.