Therapeutic Drug Monitoring
Anti-Infliximab (Remicade®)Free Drug/ADA Dual ELISA Kit
- SKU:
- HUMB00004
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free/Total Semi-quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Infliximab (Remicade®)
- Research Area:
- Anti-TNF Alpha
Description
Anti-Infliximab Free Drug/ADA Dual ELISA Kit
Enzyme-linked immunosorbent assay for the semi-quantitative determination of total and free antibodies to infliximab in serum and plasma. Free and Total Anti-Drug Antibody (ADA) monitoring gained high importance along with measuring free drug from the patient samples. In order to make these measurements simple yet more informative, we have produced our ELISA Kit to measure Free and Total anti-drug antibodies to Infliximab simultaneously on the same plate.
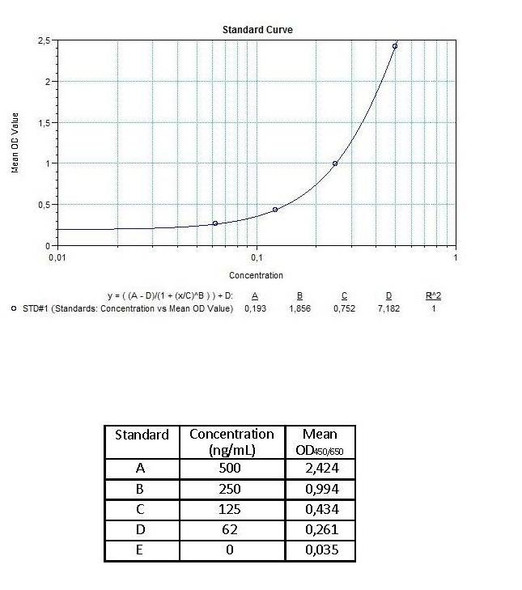
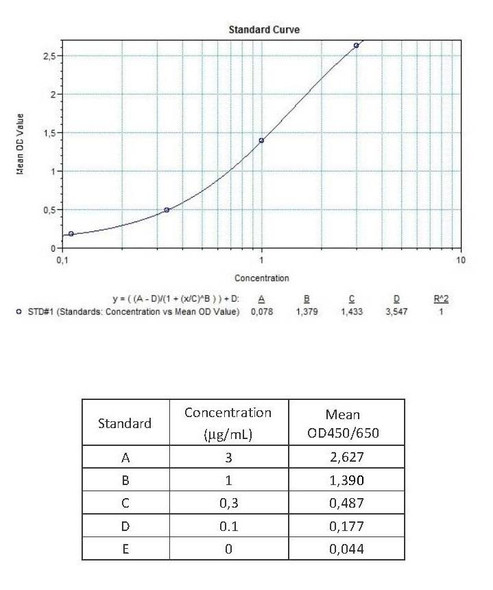
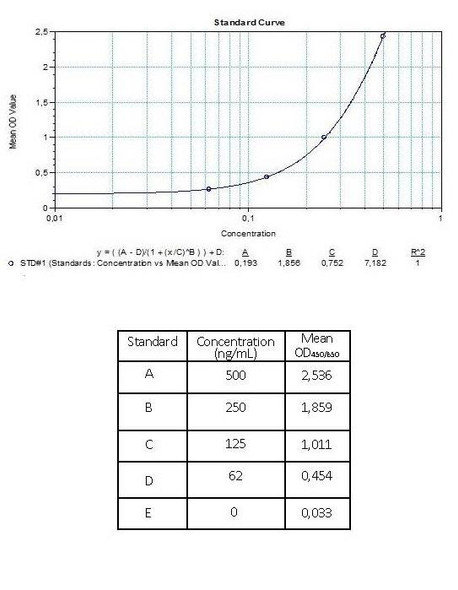
Anti-Infliximab (Remicade®) Free Drug/ADA Dual ELISA Kit test principle
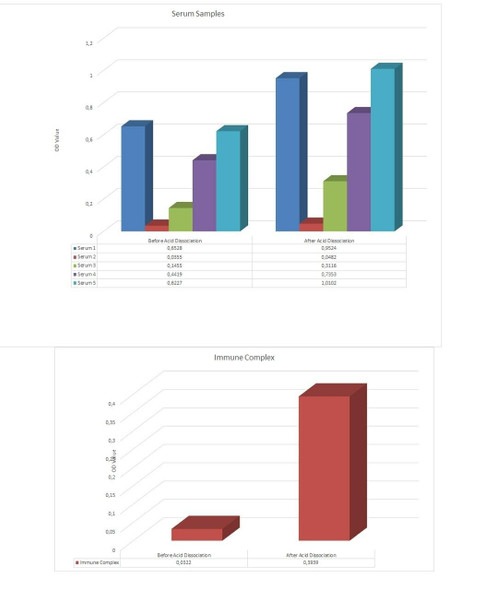
The Assay Genie Antibody to infliximab (Remicade®) ELISA is a sandwich assay for the determination of total and free antibodies against infliximab in serum and plasma samples. During the first incubation period, the separation of infiliximab specific antibody-infiliximab immune complex is provided by adding dissociation buffer.
After transferring dissociation mix to the plate, antibodies to infliximab (ATI) separated from infiliximab in patient serum/ plasma samples are captured by the drug infliximab (Remicade®) coated on the wall of the microtiter wells and peroxidase-labelled specific conjugate. After washing away the unbound components from samples, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of ATI in sample.
Assay Genie Duo ELISA kit can be also used as a semi-quantitative (screening) test in free ADA determination without dissociation and neutralization steps. Peroxidase-labelled specific conjugate and diluted serum / plasma samples are transferred simultaneously to the infliximab-coated plate and antibodies to infliximab (ATI) in patient serum/ plasma samples are captured by the drug infliximab (Remicade®) coated on the wall of the microtiter wells and peroxidase-labelled specific conjugate. After washing away the unbound components from samples, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of ATI in sample.
Anti-Infliximab Free Drug/ADA Dual ELISA Product Information
| Information | Description | |
Application | Free drug | |
Required Volume (μl) | 10 | |
Total Time (min) | 95 | |
Sample Type | Serum, Plasma | |
Number of Assays | 96 | |
Detection Limit (ng/mL) | 156 (ng/mL) | |
Spike Recovery (%) | - | |
Shelf Life (year) |
| |
Alternative Names | Tumour Necrosis Factor Alpha Remicade |
Anti-Infliximab (Remicade®) Free Drug/ADA Dual - Key Information
Infliximab mode of action
Infliximab (Remicade®) is a chimeric monoclonal antibody and used to treat autoimmune disorders. Infliximab reduces the amount of active human tumour necrosis factor alpha (hTNF alpha) in the body by binding to it and preventing it from signalling the receptors for TNF alpha on the surface of various cell types. TNF alpha is one of the key cytokines that triggers and sustains inflammatory reactions
Infliximab treatment
Infliximab (Remicade®) is used for the treatment of psoriasis, Crohn’s disease, ankylosing spondylitis, psoriatic arthritis, ulcerative colitis, and approved by FDA.
Infliximab immunogenicity
One of the major concern, despite of its wide usage, is potential development of anti-infliximab antibodies (ATI) which in turn may interfere with infliximab (Remicade®) efficacy as mainly judged by observing the relapse of signs and symptoms of disease and necessitate dose-escalation or potentially ending up the treatment.
In this context, demonstration of anti-infliximab antibodies during treatment with infliximab (Remicade®) has a major concern and monitoring for the presence and/or quantitation of specific antibodies during clinical trials is an important issue for follow up of the treatment regimens. With the Assay Genie Duo ELISA Kit infiliximab-specific antibodies (bound or free) can be detected simultaneously.
Anti-Infliximab Free Drug/ADA Dual Kit Contents
| Size | Kit Contents |
1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with infliximab. |
1 x 0.3 mL | Reactive Control |
1 x 0.5 mL | Negative Control |
1 x 0.3 mL | Immune Complex Control |
1 x 12 mL | Dissociation Buffer |
1 x 6 mL | Neutralisation Buffer Ready-to-use. |
1 x 12 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12mL | TMB Stop Solution |
1 x 50mL | Wash Buffer, concentrate (20x) Contains Buffer with Tween 20. |
2 x 1 | Adhesive Film |
1 x 12 x 8 | Microtiter Plate |
Anti-Infliximab Free Drug/ADA Dual Kit Protocol
| Steps | Protocol |
1 | Pipette 40μL of ready-to use Negative Control, Reactive Control, Immune Complex and Samples into the respective wells of dissociation plate. |
2 | Add 160μL dissociation buffer to dilution plate and incubate plate 15 min at room temperature by shaking gently. |
3 | After 15 min incubation, pipette 65μl of Peroxidase conjugate and 35μl Neutralisation Buffer into each of the wells to total antibody plate and transfer 100μl dissociation mix into each of total antibody plate wells. A1: Negative Control |
4 | If only tested for the presence of free ADA, dissociation and neutralization steps do not apply. Pipette 65μl of Peroxidase conjugate and 135μl diluted reactive control, negative control and sample into each of the wells to total antibody plate and continue to step 5. |
5 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
6 | Remove adhesive film. Discard incubation solution. Wash plate 3 times each with 300 μL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
7 | Pipette 100μL of TMB Substrate Solution into each well and incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
8 | Stop the substrate reaction by adding 100μL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow |
9 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution |
Trademarks
Remicade® is a registered trademark of Janssen Biotech Inc.