Therapeutic Drug Monitoring
Anti-Denosumab (Prolia®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00037
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Denosumab (Prolia®)
- Research Area:
- Osteoporosis
Description
Anti-Denosumab (Prolia®) ADA Qualitative ELISA Kit
Enzyme-linked immunosorbent assay for the qualitative determination (screening) of anti-Denosumab (Prolia®) antibodies in serum and plasma.The Assay Genie Antibody to Denosumab (Prolia®) ELISA Kit is intended for the qualitative determination of Denosumab anti-drug antibodies (ADA) in serum and plasma. It is for professional use only.
Anti-Denosumab (Prolia®) ADA Qualitative ELISA Kit test principle
The Assay Genie Antibody to Denosumab (Prolia®) ELISA is a sandwich assay for the determination of anti-Denosumab antibodies in serum and plasma samples. During the first incubation period, antibodies to Denosumab (ATD) in patient serum/ plasma samples are captured by the drug Denosumab (Prolia®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase-labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-Denosumab antibodies in sample.
Anti-Denosumab (Prolia®) ADA Qualitative ELISA Product Information
| Information | Description |
| Application | Free drug |
| Required Volume (uL) | 10 |
| Total Time (min) | 140 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | plus/minus |
| Spike Recovery (%) | - |
| Shelf Life (year) | 1 |
| Alternative Names | Anti-RANKL mAb Prolia |
Anti-Denosumab (Prolia®) ADA Qualitative ELISA - Key Information
Denosumab (Prolia®) mode of action
Denosumab (Prolia®) has a mechanism of action unlike other osteoporosis treatments. Administered every six months as a subcutaneous injection, Denosumab (Prolia®) is the only therapy that targets RANK Ligand, an essential regulator of osteoclasts (the cells that break down bone). Through highly specific inhibition of RANK ligand, Denosumab (Prolia®) decreases bone resorption, and improves bone density at all measured skeletal sites. Denosumab (Prolia®) is reimbursed for women and men aged 70 or over with a Bone Mineral Density (BMD) T-score of -2.5 or less.
Denosumab (Prolia®) uses
Denosumab (Prolia®) is used for the treatment of osteoporosis in postmenopausal women to reduce the risk of vertebral, non-vertebral and hip fractures and to increase bone mass in men with osteoporosis at increased risk of fracture.
Denosumab (Prolia®) treatment
Denosumab (Prolia®)is a twice-yearly injection under the skin, usually administered by a general practitioner or practice nurse, and works by targeting the cells that break down bone (osteoclasts) thereby making bone less susceptible to osteoporosis-related fractures.
Denosumab (Prolia®) and the receptor activator of nuclear factor kappa- ligand (RANKL)
Denosumab binds to RANKL, a transmembrane or soluble protein essential for the formation, function, and survival of osteoclasts, the cells responsible for bone resorption. Denosumab (Prolia®) prevents RANKL from activating its receptor, RANK, on the surface of osteoclasts and their precursors. Prevention of the RANKL/RANK interaction inhibits osteoclast formation, function, and survival, thereby decreasing bone resorption and increasing bone mass and strength in both cortical and trabecular bone.
Denosumab (Prolia®) immunogenicity
As with any biologic therapeutic, immunogenicity, in the form of anti-denosumab antibodies can occur. The demonstration of anti-denosumab antibodies during treatment with denosumab (Prolia®) is a major concern. The Assay Genie Anti-Denosumab (Prolia®) ADA Qualitative ELISA Kit can be efficiently used for monitoring anti-denosumab antibodies in biological samples and is for research use only.
Anti-Denosumab (Prolia®) ADA Qualitative ELISA Kit Contents
| Size | Kit Contents |
| 1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
| 1 x 0.25 mL | Reactive Control |
| 1 x 0.5 mL | Negative Control |
| 1 x 12 mL | Assay Buffer |
| 1 x 12 mL | Peroxidase Conjugate |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
Anti-Denosumab (Prolia®) ADA Qualitative ELISA Protocol
| Steps | Protocol |
| 1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
| 2 | QUALITATIVE ELISA TEST FORMAT Wells |
| 3 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at |
| 4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 5 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
| 6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
| 7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 8 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
| 10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
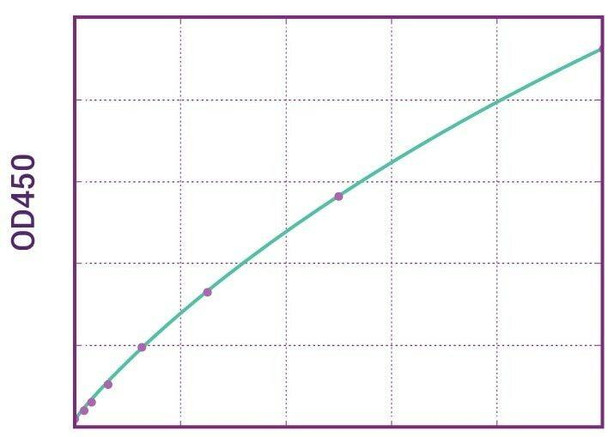
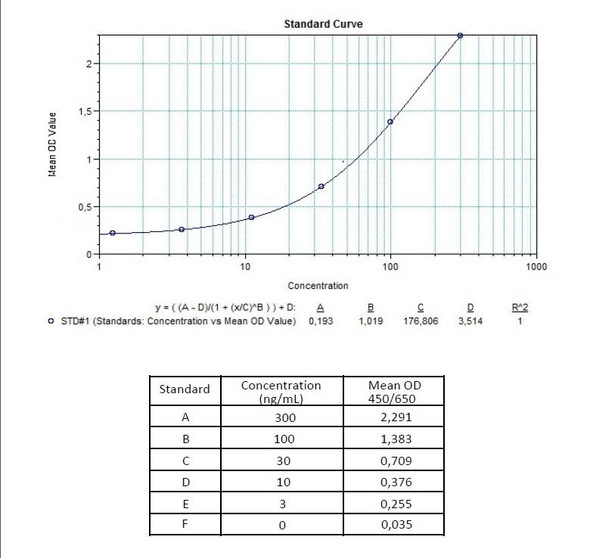
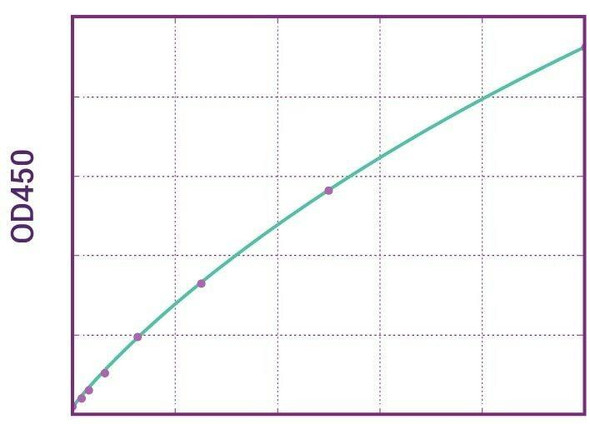
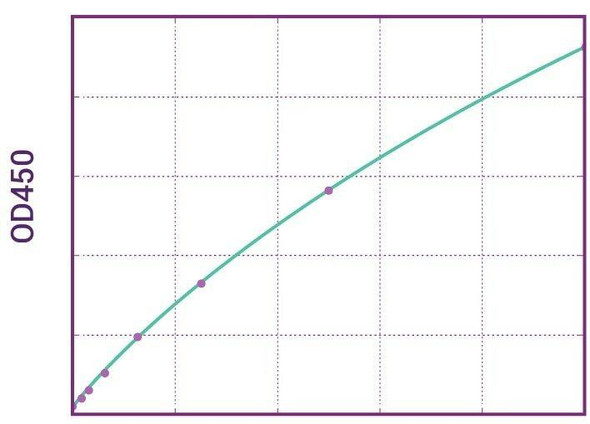
| 11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Prolia® is a trademark of Amgen Inc.