Cell Biology Antibodies 11
Anti-CRYGS Antibody (CAB7888)
- SKU:
- CAB7888
- Product Type:
- Antibody
- Reactivity:
- Human
- Reactivity:
- Mouse
- Reactivity:
- Rat
- Host Species:
- Rabbit
- Isotype:
- IgG
- Antibody Type:
- Polyclonal Antibody
- Research Area:
- Cell Biology
Description
| Antibody Name: | Anti-CRYGS Antibody |
| Antibody SKU: | CAB7888 |
| Antibody Size: | 20uL, 50uL, 100uL |
| Application: | WB |
| Reactivity: | Human, Mouse, Rat |
| Host Species: | Rabbit |
| Immunogen: | Recombinant fusion protein containing a sequence corresponding to amino acids 1-178 of human CRYGS (NP_060011.1). |
| Application: | WB |
| Recommended Dilution: | WB 1:500 - 1:2000 |
| Reactivity: | Human, Mouse, Rat |
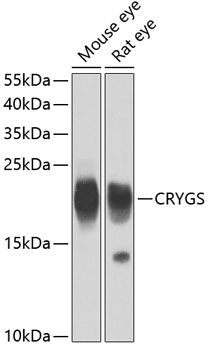
| Positive Samples: | Mouse eye, Rat eye |
| Immunogen: | Recombinant fusion protein containing a sequence corresponding to amino acids 1-178 of human CRYGS (NP_060011.1). |
| Purification Method: | Affinity purification |
| Storage Buffer: | Store at -20'C. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide, 50% glycerol, pH7.3. |
| Isotype: | IgG |
| Sequence: | MSKT GTKI TFYE DKNF QGRR YDCD CDCA DFHT YLSR CNSI KVEG GTWA VYER PNFA GYMY ILPQ GEYP EYQR WMGL NDRL SSCR AVHL PSGG QYKI QIFE KGDF SGQM YETT EDCP SIME QFHM REIH SCKV LEGV WIFY ELPN YRGR QYLL DKKE YRKP IDWG AASP AVQS FRRI VE |
| Gene ID: | 1427 |
| Uniprot: | P22914 |
| Cellular Location: | |
| Calculated MW: | 21kDa |
| Observed MW: | 21kDa |
| Synonyms: | CRYGS, CRYG8, CTRCT20 |
| Background: | Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a superfamily. Alpha and beta families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. This gene encodes a protein initially considered to be a beta-crystallin but the encoded protein is monomeric and has greater sequence similarity to other gamma-crystallins. This gene encodes the most significant gamma-crystallin in adult eye lens tissue. Whether due to aging or mutations in specific genes, gamma-crystallins have been involved in cataract formation. |
| UniProt Protein Function: | CRYGS: Crystallins are the dominant structural components of the vertebrate eye lens. Belongs to the beta/gamma-crystallin family. |
| UniProt Protein Details: | Chromosomal Location of Human Ortholog: 3q27.3 Disease: Cataract 20, Multiple Types |
| NCBI Summary: | Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a superfamily. Alpha and beta families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. This gene encodes a protein initially considered to be a beta-crystallin but the encoded protein is monomeric and has greater sequence similarity to other gamma-crystallins. This gene encodes the most significant gamma-crystallin in adult eye lens tissue. Whether due to aging or mutations in specific genes, gamma-crystallins have been involved in cataract formation. [provided by RefSeq, Jul 2008] |
| UniProt Code: | P22914 |
| NCBI GenInfo Identifier: | 4033688 |
| NCBI Gene ID: | 1427 |
| NCBI Accession: | P22914.4 |
| UniProt Secondary Accession: | P22914,B2RAF8, |
| UniProt Related Accession: | P22914 |
| Molecular Weight: | 21,007 Da |
| NCBI Full Name: | Beta-crystallin S |
| NCBI Synonym Full Names: | crystallin gamma S |
| NCBI Official Symbol: | CRYGS |
| NCBI Official Synonym Symbols: | CRYG8; CTRCT20 |
| NCBI Protein Information: | beta-crystallin S |
| UniProt Protein Name: | Beta-crystallin S |
| UniProt Synonym Protein Names: | Gamma-S-crystallin; Gamma-crystallin S |
| Protein Family: | Beta-crystallin |
| UniProt Gene Name: | CRYGS |
| UniProt Entry Name: | CRBS_HUMAN |
View AllClose