Therapeutic Drug Monitoring
Anti-Avelumab (Bavencio®)ADA Qualitative ELISA Kit
- SKU:
- HUMB00043
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Qualitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Avelumab (Bavencio®)
- Research Area:
- Checkpoint Inhibitors
Description
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA Kit
Enzyme-linked immunosorbent assay for the qualitative determination of antibodies to Avelumab in serum and plasma.The Assay Genie® Antibody to Avelumab (Bavencio®) ELISA Kit is intended for the qualitative determination of antibodies to Avelumab (Bavencio®) in serum and plasma. It is for professional use only.
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA Kit test principle
The Assay Genie® Antibody to Avelumab (Bavencio®) ELISA is a sandwich assay for the determination of antibodies against avelumab in serum and plasma samples. During the first incubation period, anti-avelumab antibodies in patient serum/ plasma samples are captured by the drug avelumab (Bavencio®) coated on the wall of the microtiter wells. After washing away the unbound components from samples, a peroxidase-labelled specific conjugate is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-avelumab antibodies in the sample.
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA Product Information
| Information | Description | |
| Application | Free drug | |
| Required Volume (μl) | 10 | |
| Total Time (min) | 140 | |
| Sample Type | Serum, Plasma | |
| Number of Assays | 96 | |
| Detection Limit (ng/mL) | plus/minus | |
| Spike Recovery (%) | - | |
| Shelf Life (year) |
| |
| Alternative Names | Anti-PD-L1 IgG1 lambda mAb |
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA - Key Information
Avelumab (Bavencio®) mode of action
Avelumab (Bavencio®) (also known as MSB0010718C) is an investigational fully human anti-PD-L1 IgG1 lambda monoclonal antibody that has a molecular weight of approximately 147 kDa. By inhibiting PD-L1 interactions, Avelumab is thought to enable the activation of T-cells and the adaptive immune system. By retaining a native Fc-region, avelumab is thought to engage the innate immune system and may induce antibody-dependent cellmediated cytotoxicity (ADCC). Importantly, avelumab has not shown antibody-dependent cell-mediated cytotoxicity against immune cell subsets in humans.
Avelumab (Bavencio®) uses
Avelumab is a programmed death ligand-1 (PD-L1) blocking antibody. It is used for the treatment of adults and pediatric patients greater than 12 years with metastatic Merkel Cell Carcinoma (MCC).
Avelumab (Bavencio®) and PD-L1
The mechanism of Action PD-L1 may be expressed on tumor cells and tumor-infiltrating immune cells and can contribute to the inhibition of the anti-tumor immune response in the tumor microenvironment. Binding of PD-L1 to the PD-1 and B7 receptors found on T cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation and cytokine production. Avelumab binds PD-L1 and blocks the interaction between PD-L1 and its receptors PD1 and B7.1. This interaction releases the inhibitory effects of PD-L1 on the immune response resulting in the restoration of immune responses, including anti-tumor immune responses.
Avelumab has also been shown to induce antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro. In syngeneic mouse tumor models, blocking PD-L1 activity resulted in decreased tumor growth.
Avelumab (Bavencio®) pharmacokinetics
The pharmacokinetics of Avelumab was studied in 1629 patients who received doses ranging from 1 to 20 mg/kg every 2 weeks. The data showed that the exposure of Avelumab increased dose-proportionally in the dose range of 10 to 20 mg/kg every 2 weeks. Steady-state concentrations of Avelumab were reached after approximately 4 to 6 weeks (2 to 3 cycles) of repeated dosing, and the systemic accumulation was approximately 1.25-fold. According to FDA's data, Cmax value was found 30 μg/mL and Cmin value was found as 22 μg/mL. In observed ADA-positive patients, the Cmin value was increased to 27.2 μg/mL, while in ADA-negative patients, this value decreased to 14.1 μg/mL. Distribution The geometric mean volume of distribution at steady state for a subject receiving 10 mg/kg was 4.72 L
The primary elimination mechanism of Avelumab is proteolytic degradation. Based on population pharmacokinetic analyses in patients with solid tumors, the total systemic clearance was 0.59 L/day and the terminal half-life was 6.1 days in patients receiving 10 mg/kg. In a post hoc analysis, avelumab clearance was found to decrease over time in patients with MCC, with a mean maximal reduction (% coefficient of variation [CV%]) from baseline value of approximately 41.7% (40.0%). Body weight was positively correlated with total systemic clearance in population pharmacokinetic analyses.
Avelumab (Bavencio®) immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to avelumab in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Of 1738 patients treated with BAVENCIO 10 mg/kg as an intravenous infusion every 2 weeks, 1558 were evaluable for treatment emergent anti-drug antibodies (ADA) and 64 (4.1%) tested positive. The development of treatment-emergent ADA against avelumab did not appear to alter the pharmacokinetic profile or risk of infusion related reactions. The use of avelumab (Bavencio®) was associated to the development of anti-avelumab antibodies, even some might be neutralizing, in various percentages of patients during therapy with the drug.
The Assay Genie Avelumab-ELISA and Anti-Avelumab ADA Qualitative ELISA Kits can be efficiently used, for monitoring serum through levels and the presence of anti-avelumab antibodies respectively, during therapy and offers the scientist a tool for decision on possible preventive measures.
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA Kit Contents
| Size | Kit Contents |
| 1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant. |
| 1 x 0.25 mL | Reactive Control |
| 1 x 0.5 mL | Negative Control |
| 1 x 12 mL | Assay Buffer |
| 1 x 12 mL | Peroxidase Conjugate |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
Anti-Avelumab (Bavencio®) ADA Qualitative ELISA Protocol
| Steps | Protocol |
| 1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
| 2 | QUALITATIVE ELISA TEST FORMAT Wells |
| 3 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
| 4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 5 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
| 6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 8 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
| 10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
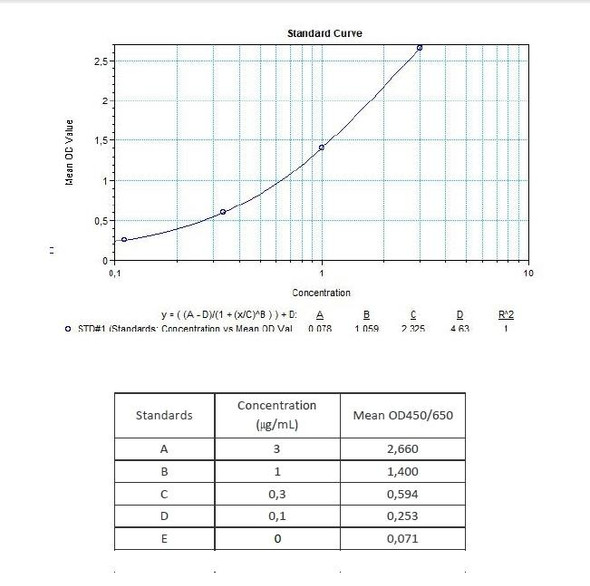
| 11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
BAVENCIO is a trademark of MERCK KGAA