Therapeutic Drug Monitoring
Anti-Adalimumab (Humira®)Free Drug/ADA Dual ELISA Kit
- SKU:
- HUMB00012
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free/Total Semi-quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Adalimumab (Humira®)
- Research Area:
- Anti-TNF Alpha
Description
Anti-Adalimumab Free Drug/ADA Dual ELISA Kit
Enzyme-linked immunosorbent assay for the semi-quantitative determination (screening) of total and free antibodies to adalimumab in serum and plasma. Free and Total Anti-Drug Antibody monitoring is of high importance, along with measuring the presence of free drug from patient samples. In order to make these measurements simple yet more informative, we have produced this ELISA Kit to measure Free and Total anti-drug antibodies to Adalimumab simultaneously on the same plate. The kit is semi-quantitative and is for research use only.
Anti-Adalimumab (Humira®) Free Drug/ADA Dual ELISA Kit test principle
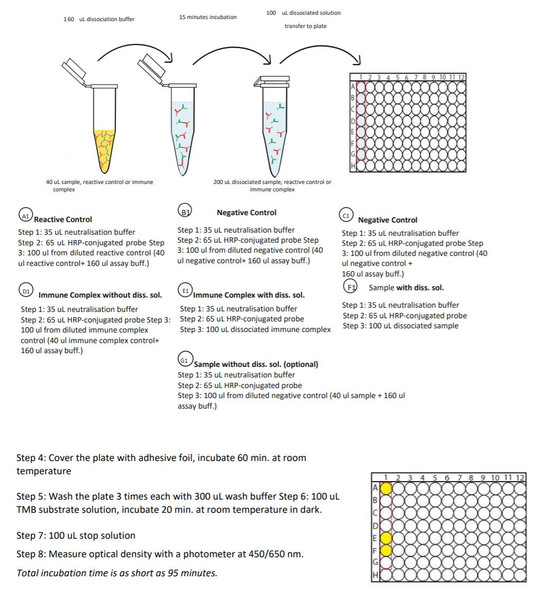
The Assay Genie Anti-Adalimumab Free Drug/ADA Dual ELISA is a sandwich assay for the determination of total antibodies against adalimumab in serum and plasma samples. During the first incubation period, the separation of adalimumab specific antibody-adalimumab immunocomplex is provided by adding dissociation buffer. After transferring dissociation mix to the plate, antibodies to adalimumab (ATA) separated from adalimumab in patient serum/ plasma samples are captured by the drug adalimumab (Humira®) coated on the wall of the microtiter wells and peroxidase-labelled specific conjugate. After washing away the unbound components from samples, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogensubstrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of ATA in sample.
The Anti-Adalimumab Free Drug/ADA Dual ELISA kit can be also used as a semi-quantitative (screening) test in free ADA determination without dissociation and neutralization steps. Peroxidase-labelled specific conjugate and diluted serum/plasma samples are transferred simultaneously to the adalimumab-coated plate and antibodies to adalimumab (ATA) in patient serum/ plasma samples are captured by the drug adalimumab (Humira®) coated on the wall of the microtiter wells and peroxidase-labelled specific conjugate. After washing away the unbound components from samples, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-adalimumab antibodies in the sample.
Anti-Adalimumab (Humira®) Product Information
| Information | Description | |
Application | Free drug | |
Required Volume (μl) | 10 | |
Total Time (min) | 95 | |
Sample Type | Serum, Plasma | |
Number of Assays | 96 | |
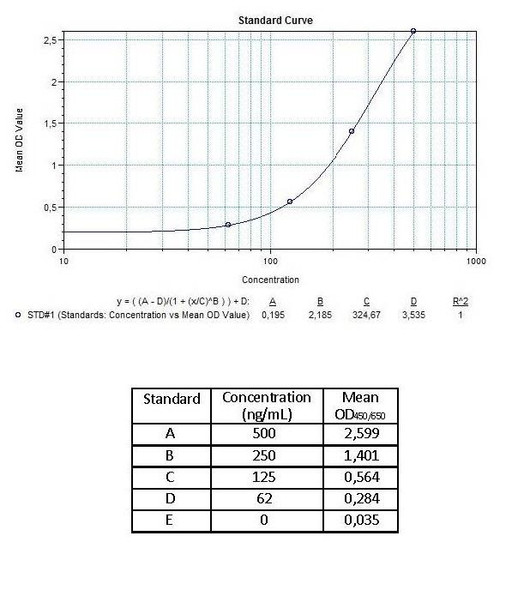
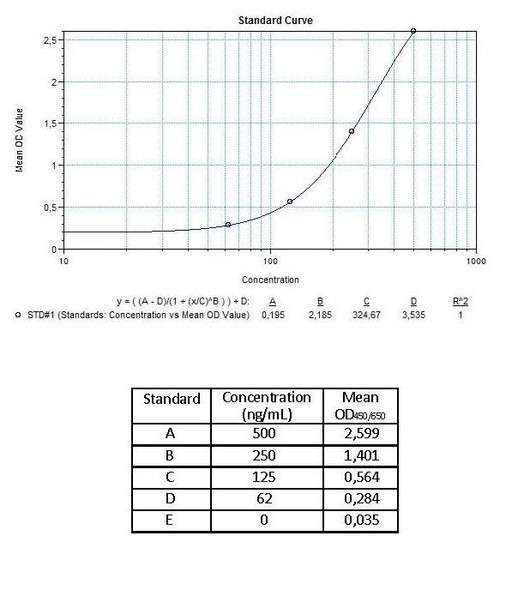
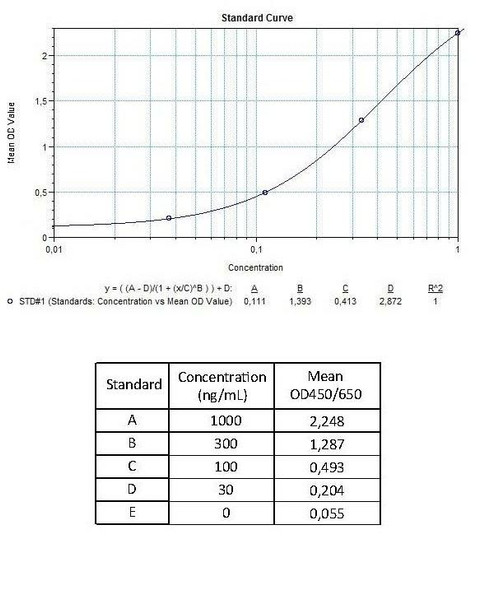
Detection Limit (ng/mL) | 250 (ng/mL) | |
Spike Recovery (%) | - | |
Shelf Life (year) |
| |
Alternative Names | Tumour Necrosis Factor Alpha Humira |
Anti-Adalimumab Free Drug/ADA Dual ELISA - Key Information
Adalimumab mode of action
Adalimumab (Humira®) is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor alpha (TNF-a). Adalimumab (Humira®) was created using phage display technology resulting in an antibody with human derived heavy and light chain variable regions and human IgG1:k constant regions. Adalimumab (Humira®) is produced by recombinant DNA technology in a mammalian cell expression system and is purified by a process that includes specific viral inactivation and removal steps. It consists of 1330 amino acids and has a molecular weight of approximately 148 kilodaltons.
Adalimumab (Humira®) binds specifically to (TNF-a) and blocks its interaction with the p55 and p75 cell surface TNF receptors. Adalimumab (Humira®) also lyses surface TNF expressing cells in vitro in the presence of complement. Adalimumab (Humira®) does not bind or inactivate lymphotoxin (TNF-beta). TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Elevated levels of TNF are found in the synovial fluid of rheumatoid arthritis, including juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis patients and play an important role in both the pathologic inflammation and the joint destruction that are hallmarks of these diseases. Increased levels of TNF are also found in psoriasis (Ps) plaques.
Adalimumab uses
Adalimumab is to treat various autoimmune diseases and works to treat inflammation. Adalimumab is administered to minimize pain and swelling due to various types of arthritis, including rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis and ankylosing spondylitis. Adalimumab also treats various skin conditions including plaque psoriasis and hidradenitis suppurativa.
Adalimumab immunogenicity
According to the manufacturer's product insert, the use of Adalimumab (Humira®) was associated to the development of anti-adalimumab antibodies, even some were reported to be neutralizing, in various percentages of patients during therapy with the drug Humira®. This could lead to severe complications. The Assay Genie Anti-Adalimumab Free Drug/ADA Dual ELISA Kit can be efficiently used for research only.
With the Assay Genie Anti-Adalimumab (Humira®) Free Drug/ADA Dual ELISA Kit adalimumab-specific antibodies that are bound to adalimumab in serum and cannot be detected by free antibody detection kits can be determined in patients receiving Humira® and free antibody to adalimumab in patient serum and plasma. This Assay Genie ELISA test can be efficiently used for monitoring adalimumab anti-drug antibodies (ADA) in biological samples and is for research use only.
Anti-Adalimumab Free Drug/ADA Dual ELISA Kit Contents
| Size | Kit Contents |
1x12x8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with infliximab. |
1 x 0.3 mL | Reactive Control |
1 x 0.5 mL | Negative Control |
1 x 0.3 mL | Immune Complex Control |
1 x 12 mL | Dissociation Buffer |
1 x 6 mL | Neutralisation Buffer Ready-to-use. |
1 x 12 mL | Assay Buffer |
1 x 12 mL | Peroxidase Conjugate |
1 x 12 mL | TMB Substrate Solution |
1 x 12mL | TMB Stop Solution |
1 x 50mL | Wash Buffer, concentrate (20x) Contains Buffer with Tween 20. |
2 x 1 | Adhesive Film |
1 x 12 x 8 | Microtiter Plate |
Anti-Adalimumab Free Drug/ADA Dual ELISA Protocol
| Steps | Protocol |
1 | Pipette 40μL of ready-to use Negative Control, Reactive Control, Immune Complex and Samples into the respective wells of dissociation plate. |
2 | Add 160μL dissociation buffer to dilution plate and incubate plate 15 min at room temperature by shaking gently. |
3 | After 15 min incubation, pipette 65μl of Peroxidase conjugate and 35μl Neutralisation Buffer into each of the wells to total antibody plate and transfer 100μl dissociation mix into each of total antibody plate wells. A1: Negative Control |
4 | If only tested for the presence of free ADA, dissociation and neutralization steps do not apply. Pipette 65μl of Peroxidase conjugate and 135μl diluted reactive control, negative control and sample into each of the wells to total antibody plate and continue to step 5. |
5 | Cover the plate with adhesive film. Briefly mix contents by gently shaking the plate. Incubate 60 min at room temperature (18-25°C). |
6 | Remove adhesive film. Discard incubation solution. Wash plate 3 times each with 300 μL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
7 | Pipette 100μL of TMB Substrate Solution into each well and incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
8 | Stop the substrate reaction by adding 100μL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow |
9 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution |
Trademarks
Humira® is a registered trademark of AbbVie Biotechnology, Inc.