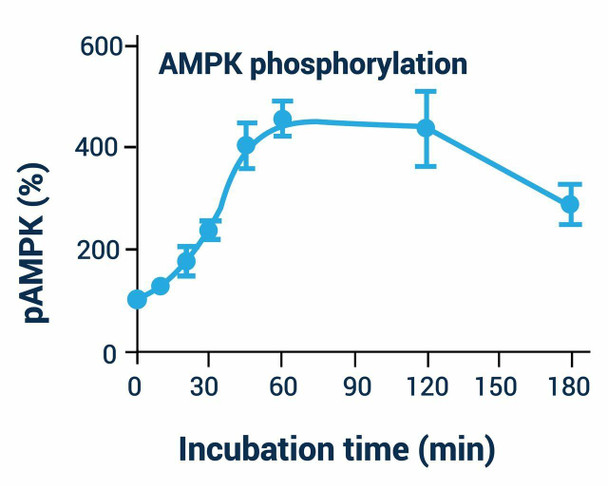
AMPK Phosphorylation Assay Kit - Information
Assay Genie's cell-based ELISA measures phosphorylated AMPK in whole cells and normalizes the signal to the total protein content. The antibody recognizes both alpha-subunits and, thus, can be used for cells from all tissues (human, mouse, rat). This simple and efficient assay eliminates the need for cell lysate preparation and can be used to study AMPK regulation in short-term and long-term assays. In this assay, cells grown in 96-well plates are fixed and permeabilized in the wells. AMPK phosphorylation (pAMPK) is measured using a fluorescent ELISA followed by total protein measurement in each well.
Applications
For quantitative fluorescent immunoenzymatic assay of AMPK phosphorylation status in cultured cells.
AMPK Phosphorylation Assay Kit - Key Features
- Sensitive. Can measure pAMPK modulation in as little as 500 cells/well.
- Simple and convenient. No cell lysis necessary, cells can be cultured for several days.
AMPK Phosphorylation Assay Kit - Data Sheet | |
| Kit Includes | 10x Wash Buffer: 25 mL Blocking Buffer: 25 mL Protein Stain: 6 mL HRP Substrate: 6 mL pAMPK-Ab1 10 uL HRP-Ab2 10 uL |
| Kit Requires | 37% formaldehyde, 3% H2O2 , black (clear bottom) cell culture 96-well plate, plate sealers: deionized or distilled water; pipetting devices; cell culture incubators; centrifuge tubes; fluorescence plate reader capable of reading at ex/em = 530/585nm and at ex/em =360/450nm |
| Method of Detection | FL360/450nm, 530/585nm |
| Samples | Cell, tissue etc |
| Species | Human, mouse, rat |
| Protocol Length | 8 hrs |
| Size | 100 tests |
| Storage | Store all reagents at -20°C |
| Shelf Life | 6 months |
More Details
The
5-AMP-activated protein kinase (AMPK) is a key sensor of intracellular energy balance. AMPK is activated in response to an increase in the AMP/ATP ratio which can be caused by a number of factors such as muscle contraction, starvation, or hypoxia. AMPK is a heterotrimeric protein complex comprising of alpha- (63 kDa), beta- (38 kDa) and beta- (38 kDa) subunits. For each subunit, isoforms have been identified (alpha1, alpha2, 1, 2, 1, 2, 3) which theoretically allow the formation of 12 different proteins. The alpha-subunit contains a serine/threonine kinase domain and the regulatory subunits contain binding sites for AMP and ATP (beta-subunit) and for glycogen (beta-subunit). AMPK is activated by phosphorylation on Thr-172 within the catalytic domain. AMP binding results in a 2 to 5-fold increase in AMPK activity compared to the basal level. Binding of AMP to the beta-subunit causes allosteric activation of the kinase and induces a conformational change in the kinase domain that protects AMPK from dephosphorylation of Thr-172.