Therapeutic Drug Monitoring
Adalimumab (Humira®) ELISA Kit
- SKU:
- HUMB00009
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Adalimumab (Humira®)
- Research Area:
- Anti-TNF Alpha
Description
Adalimumab (Humira®) ELISA Kit
Enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of free adalimumab (Humira®) in serum and plasma with confirmation. The Assay Genie adalimumab ELISA has been especially developed for the quantitative analysis of free adalimumab in serum and plasma samples.
Adalimumab (Humira®) ELISA Kit test principle
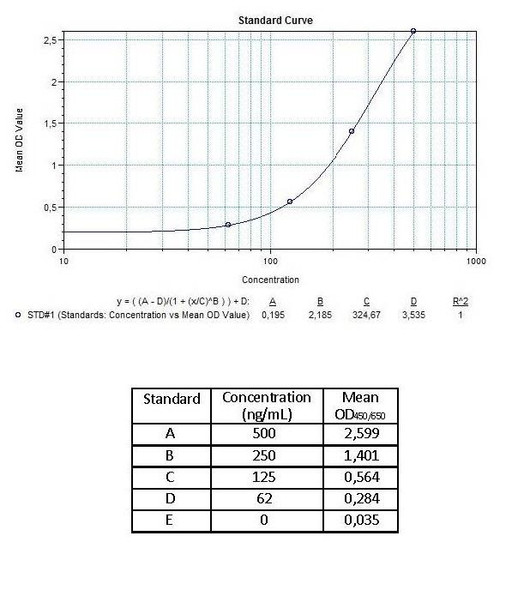
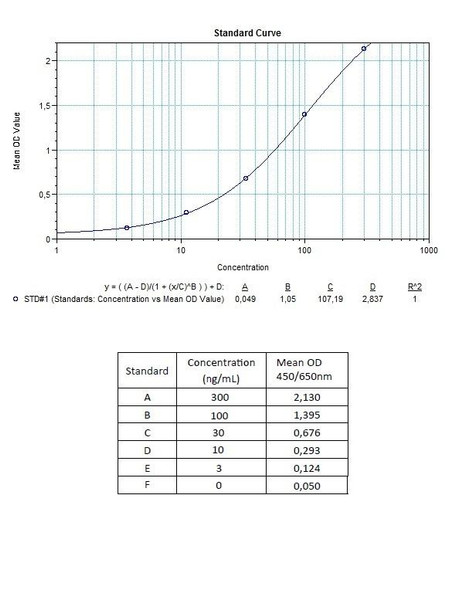
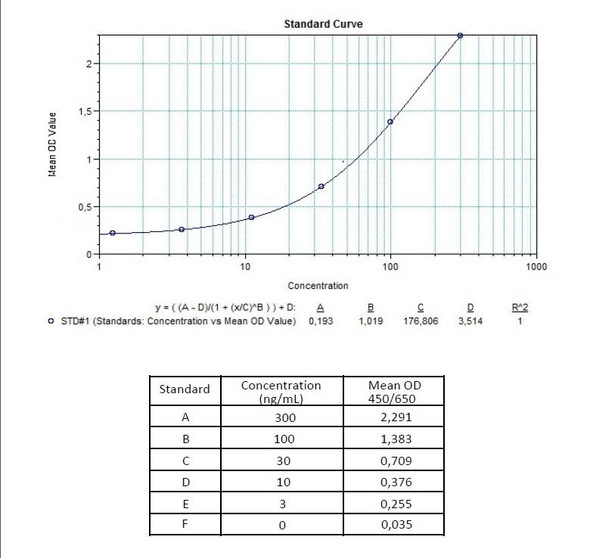
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and diluted samples (serum or plasma) are incubated in the microtitre plate coated with the reactant for adalimumab (Humira®). After incubation, the wells are washed. A horse radish peroxidase (HRP) conjugated probe is added and binds to adalimumab (Humira®) captured by the reactant on the surface of the wells. Following incubation, wells are washed, and the bound enzymatic activity is detected by addition of chromogen-substrate. The colour developed is proportional to the amount of adalimumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Adalimumab (Humira®) Product Information
| Information | Description |
Application | Free drug |
Required Volume (uL) | 10 |
Total Time (min) | 70 |
Sample Type | Serum, Plasma |
Number of Assays | 96 |
Detection Limit (ng/mL) | 30 (ng/mL) |
Spike Recovery (%) | 85-115% |
Shelf Life (year) | 1 |
Alternative Names | Tumour Necrosis Factor Alpha Humira |
Adalimumab (Humira®) - Key Information
Adalimumab mode of action
Adalimumab (Humira®) is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor alpha (TNF-a). Adalimumab (Humira®) was created using phage display technology resulting in an antibody with human derived heavy and light chain variable regions and human IgG1:k constant regions. Adalimumab (Humira®) is produced by recombinant DNA technology in a mammalian cell expression system and is purified by a process that includes specific viral inactivation and removal steps. It consists of 1330 amino acids and has a molecular weight of approximately 148 kilodaltons.
Adalimumab (Humira®) binds specifically to (TNF-a) and blocks its interaction with the p55 and p75 cell surface TNF receptors. Adalimumab (Humira®) also lyses surface TNF expressing cells in vitro in the presence of complement. Adalimumab (Humira®) does not bind or inactivate lymphotoxin (TNF-beta). TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Elevated levels of TNF are found in the synovial fluid of rheumatoid arthritis, including juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis patients and play an important role in both the pathologic inflammation and the joint destruction that are hallmarks of these diseases. Increased levels of TNF are also found in psoriasis (Ps) plaques.
Adalimumab uses
Adalimumab is to treat various autoimmune diseases and works to treat inflammation. Adalimumab is administered to minimize pain and swelling due to various types of arthritis, including rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis and ankylosing spondylitis. Adalimumab also treats various skin conditions including plaque psoriasis and hidradenitis suppurativa.
Adalimumab pharmacokinetics
After treatment with adalimumab (Humira®), a decrease in levels of acute phase reactants of inflammation (Creactive protein [CRP] and erythrocyte sedimentation rate [ESR]) and serum cytokines (IL-6) was observed compared to baseline in patients with rheumatoid arthritis. A decrease in CRP levels was also observed in patients with Crohn's disease. Serum levels of matrix metalloproteinases (MMP-1 and MMP-3) that produce tissue remodeling responsible for cartilage destruction were also decreased after adalimumab (Humira®) administration. According to the prospectus; the maximum serum concentration (Cmax) and the time to reach the maximum concentration (Tmax) were 4.7 ± 1.6 ?g/mL and 131 ± 56 hours respectively, following a single 40 mg subcutaneous administration of adalimumab (Humira®) to healthy adult subjects.
The average absolute bioavailability of adalimumab (Humira®) estimated from three studies following a single 40 mg subcutaneous dose was 64%. The pharmacokinetics of adalimumab were linear over the dose range of 0.5 to 10.0 mg/kg following a single intravenous dose. The single dose pharmacokinetics of adalimumab in rheumatoid arthritis (RA) patients were determined in several studies with intravenous doses ranging from 0.25 to 10 mg/ kg. The distribution volume (Vss) ranged from 4.7 to 6.0 L. The systemic clearance of adalimumab is approximately 12 mL/hr. The mean terminal half-life was approximately 2 weeks, ranging from 10 to 20 days across studies. Adalimumab concentrations in the synovial fluid from five rheumatoid arthritis patients ranged from 31 to 96% of those in serum. In RA patients receiving 40 mg adalimumab (Humira®) every other week, adalimumab mean steady-state trough concentrations of approximately 5 mg/mL and 8 to 9 mg/mL, were observed without and with 3 methotrexate (MTX), respectively.
Adalimumab treatment
Serum concentration of adalimumab (Humira®) might be related to predict some clinical outcome during maintenance therapy. It was also possible that the surveillance of circulating adalimumab (Humira®) concentration during maintenance therapy represents a direct and/or indirect factor for some other side effects. In this context, identification of biomarkers for (non-)response and risk factors for adverse drug reactions that might be related to serum concentrations and maintaining the effective minimum concentration of adalimumab (Humira®) in order to potentially avoid some side effects with a reliable method might be beneficial.
Adalimumab (Humira®) ELISA Kit Contents
| Size | Kit Contents |
1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
7 x 0.3 mL | Infliximab Standards A-E, High Level Control, Low Level Control |
1 x 50 mL | Assay Buffer |
1 x 12 mL | Horse radish peroxidase-Conjugated Probe. Red coloured. Ready to use. Contains HRP-probe, stabilizer and preservatives. |
1 x 12 mL | TMB Substrate Solution |
1 x 12 mL | TMB Stop Solution |
1 x 50 mL | Wash Buffer concentrate (20x) |
2 x 1 | Adhesive Foil |
Adalimumab (Humira®) ELISA Protocol
| Steps | Protocol |
1 | Pipette 100µl of Assay Buffer non-exceptionally into each of the wells to be used. |
2 | Pipette 20 µL of each ready-to use Standards, High Level Control, Low Level Control and Diluted Samples into the respective wells of microtiter plate. Wells |
3 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
5 | Pipette 100 µL of ready-to use HRP-Conjugated Probe into each well. |
6 | Cover the plate with adhesive foil. Incubate 30 min at room temperature (18- 25°C). |
7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
8 | Pipette 100 µL of TMB Substrate Solution into each well. |
9 | Incubate 10 min (without adhesive foil) at room temperature (18-25°C) in the dark |
10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
Humira® is a registered trademark of AbbVie Biotechnology, Inc.