GLRX1 has a glutathione-disulfide oxidoreductase activity in the presence of nadph and glutathione reductase. reduces low molecular weight disulfides and proteins.Glutaredoxin is a glutathione (GSH)-dependent hydrogen donor for ribonucleotide reductase and also catalyzes glutathione-disulfide oxidoreduction reactions in the presence of NADPH and glutathione reductase. GLRX1 is multifunctional enzyme with glutathione-dependent oxidoreductase, glutathione peroxidase and glutathione S-transferase (GST) activity. The disulfide bond functions as an electron carrier in the glutathione-dependent synthesis of deoxyribonucleotides by the enzyme ribonucleotide reductase. In addition, it is also involved in reducing cytosolic protein- and non-protein-disulfides in a coupled system with glutathione reductase. Required for resistance to reactive oxygen species (ROS) by directly reducing hydroperoxides and for the detoxification of ROS-mediated damage.
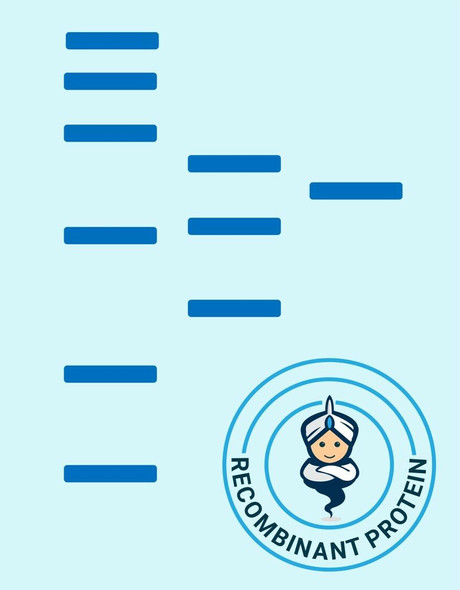
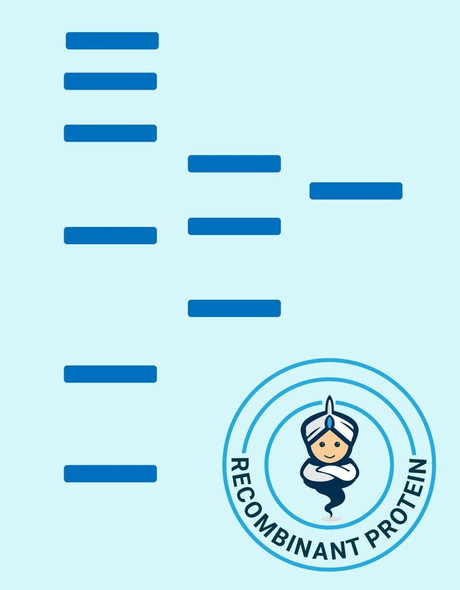
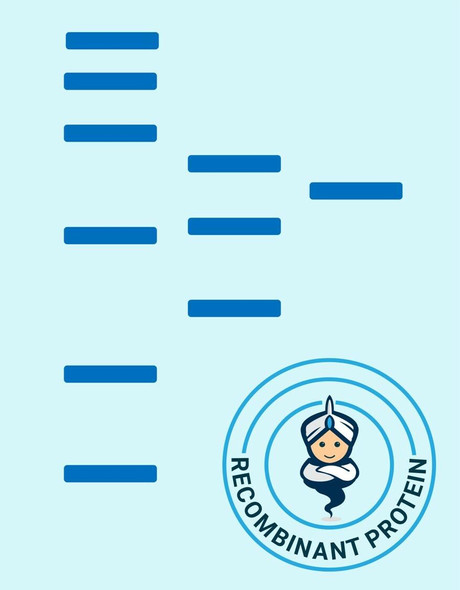
Glutaredoxin Saccharamyces cerevisiae Recombinant containing 6x His tag at C-Terminus produced in E.Coli is a single, non-glycosylated, Polypeptide chain having a molecular mass of 16 kDa.