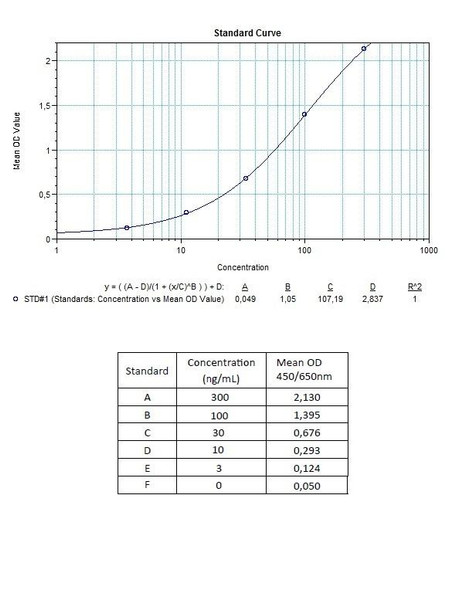
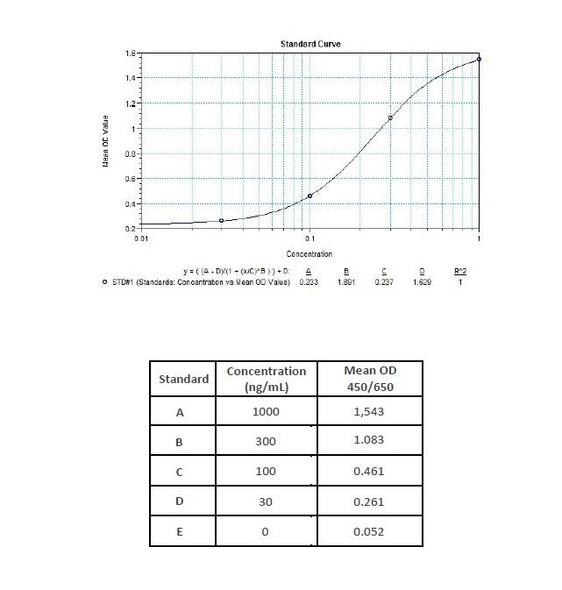
Therapeutic Drug Monitoring
Tocilizumab (Actemra®) ELISA Kit
- SKU:
- HUMB00050
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Tocilizumab (Actemra®)
- Research Area:
- Anti-Inflammatory
Description
Tocilizumab (Actemra®) ELISA Kit
Enzyme immunoassay for the quantitative determination of Tocilizumab (Actemra®) in serum and plasma. The Assay Genie Tocilizumab ELISA has been specifically developed for the quantitative analysis of Tocilizumab in serum and plasma samples. It is for professional use only.
Product Information
| Component | Amount |
| Application | Free drug |
| Required Volume (μl) | 100 |
| Total Time (min) | 70 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | 10 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
About Tocilizumab (Actemra®)
Tocilizumab is a recombinant, humanized, anti-human interleukin 6 (IL-6) receptor monoclonal antibody that achieves a significant therapeutic response rate. The light chain is made up of 214 amino acids. The heavy chain is made up of 448 amino acids. The four polypeptide chains are linked intra- and inter-molecularly by disulfide bonds. FDA approved on January 8, 2010.
Tocilizumab (injection) was further approved by the FDA for the treatment of adults with giant cell arteritis, an inflammation of the blood vessels (vasculitis) in May, 2017. In a double-blind, placebo-controlled study, the patients achieved sustained remission from Week 12 through Week 52, which was associated with significant improvements in symptoms of giant cell arteritis, normalization of inflammatory laboratory tests and tapering the use of corticosteroids.
Interleukin (IL)-6 plays essential roles not only in the immune response, but also in haematopoiesis and the central nervous system. Unregulated production of IL-6 has been found in chronic inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), systemic onset juvenile idiopathic arthritis (soJIA), Crohn's disease (CD), systemic lupus erythematosus (SLE) and vasculitis. Furthermore, IL-6 activities can explain many symptoms of these diseases. More importantly, serum levels of IL-6 are correlated with disease activity. Tocilizumab binds specifically to both soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R), and has been shown to inhibit IL-6-mediated signaling through these receptors.
Therapeutic drug monitoring (TDM) is the clinical practice of measuring specific drugs at designated intervals to maintain a constant concentration in a patient's bloodstream, thereby optimizing individual dosage regimens. The indications for drug monitoring include efficacy, compliance, drug-drug interactions, toxicity avoidance, and therapy cessation monitoring. Additionally, TDM can help to identify problems with medication compliance among noncompliant patient cases.
Biologic medicinal products (biologics) have transformed treatment landscapes worldwide for patients with hematological or solid malignancies with the 21st century. Today, as data exclusivity periods of first wave biologics approach expiration/have expired, several biosimilar products (i.e. biologics that are considered to be similar in terms of quality, safety and efficacy to an approved reference biologic) are being developed or have already been approved for human use.
Like all biologics, biosimilars are structurally complex proteins that are typically manufactured using genetically engineered animal, bacterial or plant cell culture systems. As a consequence of this molecular complexity and the proprietary nature of the manufacturing process, which will inevitably result in the use of different host cell lines and expression systems as well as related differences in manufacturing conditions, it is not possible to manufacture exact copies of a reference biologic.
When administered to patients, all therapeutic proteins have the potential to induce an unwanted immune response (i.e. to stimulate the formation of antidrug antibodies [ADAs]). The impact of immune responses can range from no apparent effect to changes in pharmacokinetics, loss of effect and serious adverse events. Furthermore, the immunogenicity profile of a biologic can be significantly altered by even small differences in its manufacturing process that are accompanied by a change in product attributes, as well as differences in dosing schedules, administration routes or patient populations.
Tocilizumab (Actemra®) Test Principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the reactant for tocilizumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to tocilizumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of tocilizumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Trademarks
Tocilizumab is a registered trademark of Chugai Pharmaceuticals, a member of the Roche Group.