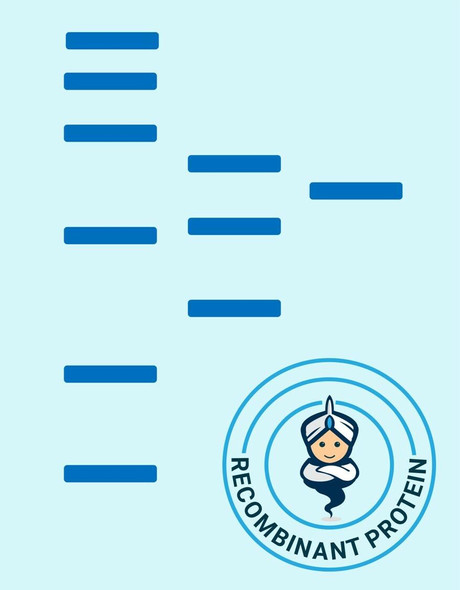
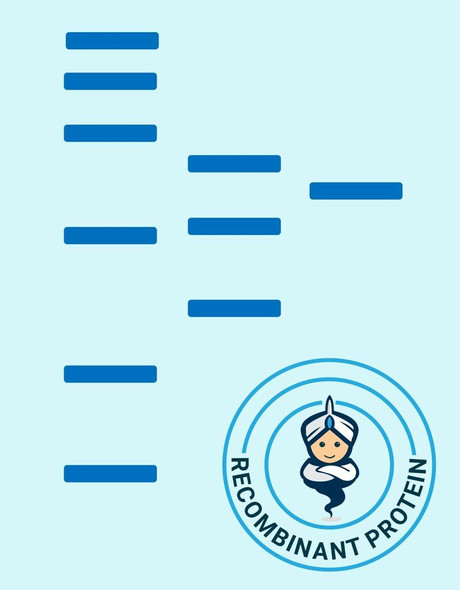
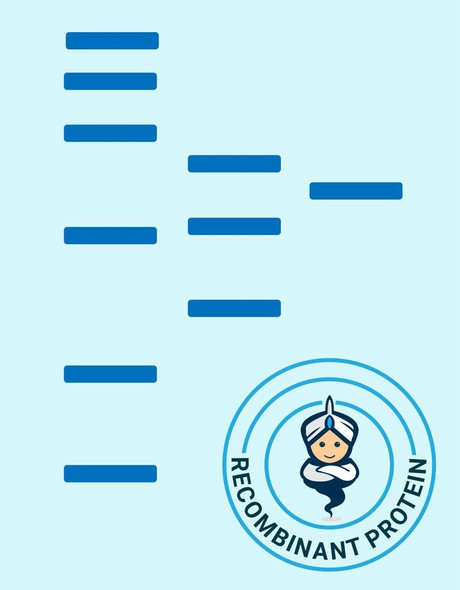
Matrix metalloproteinase-7 (MMP-7) also known as matrilysin and PUMP (EC 3.4.24.23) cleaves a number of substrates including collagen types IV and X, elastin, fibronectin, gelatin, laminin and proteoglycans. MMP-7 is closely related to the stromelysin family members but is encoded by a different gene. MMP-7 is the smallest of all the MMPs consisting of a pro-peptide domain and a catalytic domain. It lacks the hemopexin-like domain common to other members of the MMPs. MMP-7 is secreted as a 28 kDa proenzyme and can be activated in vitro by organomercurials and trypsin and in vivo by MMP-3 to a 18 kDa active MMP-7 enzyme. Once activated, MMP-7 can activate pro-MMP-1 and pro-MMP-9 but not pro-MMP-2. MMP-7 is widely expressed having been reported in elevated levels in cycling endometrium as well as in colorectal cancers and adenomas, hepatocellular carcinomas, rectal carcinomas, and approximately 50% of gliomas.