Cell Cycle Antibodies 2
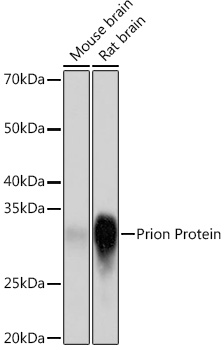
Anti-Prion Protein Antibody (CAB8821)
- SKU:
- CAB8821
- Product Type:
- Antibody
- Reactivity:
- Human
- Reactivity:
- Mouse
- Reactivity:
- Rat
- Host Species:
- Rabbit
- Isotype:
- IgG
- Antibody Type:
- Monoclonal Antibody
- Research Area:
- Cell Cycle
Description
| Antibody Name: | Anti-Prion Protein Antibody |
| Antibody SKU: | CAB8821 |
| Antibody Size: | 20uL, 50uL, 100uL |
| Application: | WB |
| Reactivity: | Human, Mouse, Rat |
| Host Species: | Rabbit |
| Immunogen: | A synthesized peptide derived from human Prion Protein |
| Application: | WB |
| Recommended Dilution: | WB 1:500 - 1:2000 |
| Reactivity: | Human, Mouse, Rat |
| Positive Samples: | Mouse brain, Rat brain |
| Immunogen: | A synthesized peptide derived from human Prion Protein |
| Purification Method: | Affinity purification |
| Storage Buffer: | Store at -20'C. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide, 0.05% BSA, 50% glycerol, pH7.3. |
| Isotype: | IgG |
| Sequence: | Email for sequence |
| Gene ID: | 5621 |
| Uniprot: | P04156 |
| Cellular Location: | |
| Calculated MW: | 28kDa |
| Observed MW: | 30KDa |
| Synonyms: | ASCR, AltPrP, CD230, CJD, GSS, KURU, PRIP, PrP, PrP27-30, PrP33-35C, PrPc, p27-30 |
| Background: | The protein encoded by this gene is a membrane glycosylphosphatidylinositol-anchored glycoprotein that tends to aggregate into rod-like structures. The encoded protein contains a highly unstable region of five tandem octapeptide repeats. This gene is found on chromosome 20, approximately 20 kbp upstream of a gene which encodes a biochemically and structurally similar protein to the one encoded by this gene. Mutations in the repeat region as well as elsewhere in this gene have been associated with Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstmann-Straussler disease, Huntington disease-like 1, and kuru. An overlapping open reading frame has been found for this gene that encodes a smaller, structurally unrelated protein, AltPrp. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Nov 2014] |
| UniProt Protein Function: | PRNP: May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains. PrP is found in high quantity in the brain of humans and animals infected with neurodegenerative diseases known as transmissible spongiform encephalopathies or prion diseases, like: Creutzfeldt-Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann-Straussler disease (GSD), Huntington disease-like type 1 (HDL1) and kuru in humans; scrapie in sheep and goat; bovine spongiform encephalopathy (BSE) in cattle; transmissible mink encephalopathy (TME); chronic wasting disease (CWD) of mule deer and elk; feline spongiform encephalopathy (FSE) in cats and exotic ungulate encephalopathy (EUE) in nyala and greater kudu. The prion diseases illustrate three manifestations of CNS degeneration: (1) infectious (2) sporadic and (3) dominantly inherited forms. TME, CWD, BSE, FSE, EUE are all thought to occur after consumption of prion-infected foodstuffs. Defects in PRNP are the cause of Creutzfeldt-Jakob disease (CJD). CJD occurs primarily as a sporadic disorder (1 per million), while 10-15% are familial. Accidental transmission of CJD to humans appears to be iatrogenic (contaminated human growth hormone (HGH), corneal transplantation, electroencephalographic electrode implantation, etc.). Epidemiologic studies have failed to implicate the ingestion of infected annimal meat in the pathogenesis of CJD in human. The triad of microscopic features that characterize the prion diseases consists of (1) spongiform degeneration of neurons, (2) severe astrocytic gliosis that often appears to be out of proportion to the degree of nerve cell loss, and (3) amyloid plaque formation. CJD is characterized by progressive dementia and myoclonic seizures, affecting adults in mid-life. Some patients present sleep disorders, abnormalities of high cortical function, cerebellar and corticospinal disturbances. The disease ends in death after a 3-12 months illness. Defects in PRNP are the cause of fatal familial insomnia (FFI). FFI is an autosomal dominant disorder and is characterized by neuronal degeneration limited to selected thalamic nuclei and progressive insomnia. Defects in PRNP are the cause of Gerstmann-Straussler disease (GSD). GSD is a heterogeneous disorder and was defined as a spinocerebellar ataxia with dementia and plaquelike deposits. GSD incidence is less than 2 per 100 million live births. Defects in PRNP are the cause of Huntington disease-like type 1 (HDL1). HDL1 is an autosomal dominant, early onset neurodegenerative disorder with prominent psychiatric features. Defects in PRNP are the cause of kuru (KURU). Kuru is transmitted during ritualistic cannibalism, among natives of the New Guinea highlands. Patients exhibit various movement disorders like cerebellar abnormalities, rigidity of the limbs, and clonus. Emotional lability is present, and dementia is conspicuously absent. Death usually occurs from 3 to 12 month after onset. Defects in PRNP are the cause of spongiform encephalopathy with neuropsychiatric features (SENF); an autosomal dominant presenile dementia with a rapidly progressive and protracted clinical course. The dementia was characterized clinically by frontotemporal features, including early personality changes. Some patients had memory loss, several showed aggressiveness, hyperorality and verbal stereotypy, others had parkinsonian symptoms. Belongs to the prion family. 2 isoforms of the human protein are produced by alternative initiation. |
| UniProt Protein Details: | Protein type:Microtubule-binding; Membrane protein, GPI anchor Chromosomal Location of Human Ortholog: 20p13 Cellular Component: Golgi apparatus; mitochondrial outer membrane; extrinsic to membrane; cell surface; endoplasmic reticulum; cytoplasm; plasma membrane; integral to membrane; nucleus; lipid raft Molecular Function:tubulin binding; ATP-dependent protein binding; identical protein binding; protein binding; copper ion binding; chaperone binding; microtubule binding Biological Process: axon guidance; cellular copper ion homeostasis; metabolic process; negative regulation of activated T cell proliferation; negative regulation of transcription factor activity; negative regulation of T cell receptor signaling pathway; negative regulation of interleukin-2 production; learning and/or memory; regulation of protein localization; negative regulation of interleukin-17 production; response to cadmium ion; negative regulation of protein amino acid phosphorylation; negative regulation of interferon-gamma production; response to oxidative stress; cell cycle arrest; protein homooligomerization; negative regulation of apoptosis Disease: Gerstmann-straussler Disease; Huntington Disease-like 1; Kuru, Susceptibility To; Fatal Familial Insomnia; Spongiform Encephalopathy With Neuropsychiatric Features; Creutzfeldt-jakob Disease |
| NCBI Summary: | The protein encoded by this gene is a membrane glycosylphosphatidylinositol-anchored glycoprotein that tends to aggregate into rod-like structures. The encoded protein contains a highly unstable region of five tandem octapeptide repeats. This gene is found on chromosome 20, approximately 20 kbp upstream of a gene which encodes a biochemically and structurally similar protein to the one encoded by this gene. Mutations in the repeat region as well as elsewhere in this gene have been associated with Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstmann-Straussler disease, Huntington disease-like 1, and kuru. An overlapping open reading frame has been found for this gene that encodes a smaller, structurally unrelated protein, AltPrp. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Nov 2014] |
| UniProt Code: | P04156 |
| NCBI GenInfo Identifier: | 130912 |
| NCBI Gene ID: | 5621 |
| NCBI Accession: | P04156.1 |
| UniProt Secondary Accession: | P04156,O60489, P78446, Q15216, Q15221, Q27H91, Q5QPB4 Q8TBG0, Q96E70, Q9UP19, |
| UniProt Related Accession: | P04156,F7VJQ1 |
| Molecular Weight: | 253 |
| NCBI Full Name: | Major prion protein |
| NCBI Synonym Full Names: | prion protein |
| NCBI Official Symbol: | PRNP |
| NCBI Official Synonym Symbols: | CJD; GSS; PrP; ASCR; KURU; PRIP; PrPc; CD230; AltPrP; p27-30; PrP27-30; PrP33-35C |
| NCBI Protein Information: | alternative prion protein; major prion protein; CD230 antigen; prion-related protein |
| UniProt Protein Name: | Major prion protein |
| UniProt Synonym Protein Names: | ASCR; PrP27-30; PrP33-35C; CD_antigen: CD230 |
| Protein Family: | Major prion protein |
| UniProt Gene Name: | PRNP |
| UniProt Entry Name: | PRIO_HUMAN |